BMP signaling induces astrocytic differentiation of clinically derived oligodendroglioma propagating cells
- PMID: 24269952
- PMCID: PMC4006982
- DOI: 10.1158/1541-7786.MCR-13-0349
BMP signaling induces astrocytic differentiation of clinically derived oligodendroglioma propagating cells
Abstract
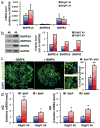
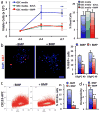
Oligodendrogliomas are a type of glioma that lack detailed investigation because of an inability to cultivate oligodendroglioma cells that faithfully recapitulate their salient qualities. We have successfully isolated and propagated glioma stem-like cells from multiple clinical oligodendroglioma specimens. These oligodendroglioma-propagating cells (OligPC) are multipotent and form xenografts with oligodendroglioma features. Bone morphogenetic proteins (BMP) are considered potent inhibitors of oligodendrogliogenesis during development; therefore, the effects of BMP signaling in OligPCs were characterized. BMP pathway components are expressed by OligPCs and canonical signaling via Smad proteins is intact. This signaling potently depletes CD133-positive OligPCs, decreasing proliferation, and inducing astrocytic differentiation. Furthermore, analyses revealed that cytoplasmic sequestration of the oligodendrocyte differentiation factors OLIG1/2 by the BMP signaling effectors ID2 and ID4 is a plausible underlying mechanism. These findings elucidate the molecular pathways that underlie the effects of BMP signaling on oligodendroglioma stem-like cells.
Implications: Stem-like cells are capable of propagating oligodendrogliomas, and BMP signaling potently diminishes their stemness by inducing astrocytic differentiation, suggesting that BMP activation may be effective as a cancer stem cell-targeted therapy.
Conflict of interest statement
The authors disclose no potential conflicts of interest.
Figures





References
-
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. - PubMed
-
- Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer research. 2004;64:7011–21. - PubMed
-
- Das S, Srikanth M, Kessler JA. Cancer stem cells and glioma. Nat Clin Pract Neurol. 2008;4:427–35. - PubMed
-
- Shih AH, Dai C, Hu X, Rosenblum MK, Koutcher JA, Holland EC. Dose-dependent effects of platelet-derived growth factor-B on glial tumorigenesis. Cancer research. 2004;64:4783–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

