Platelet gene therapy corrects the hemophilic phenotype in immunocompromised hemophilia A mice transplanted with genetically manipulated human cord blood stem cells
- PMID: 24269957
- PMCID: PMC3894495
- DOI: 10.1182/blood-2013-08-520478
Platelet gene therapy corrects the hemophilic phenotype in immunocompromised hemophilia A mice transplanted with genetically manipulated human cord blood stem cells
Abstract
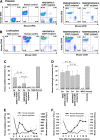
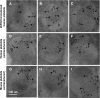
Our previous studies have demonstrated that platelet FVIII (2bF8) gene therapy can improve hemostasis in hemophilia A mice, even in the presence of inhibitory antibodies, but none of our studies has targeted human cells. Here, we evaluated the feasibility for lentivirus (LV)-mediated human platelet gene therapy of hemophilia A. Human platelet FVIII expression was introduced by 2bF8LV-mediated transduction of human cord blood (hCB) CD34(+) cells followed by xenotransplantation into immunocompromised NSG mice or NSG mice in an FVIII(null) background (NSGF8KO). Platelet FVIII was detected in all recipients that received 2bF8LV-transduced hCB cells as long as human platelet chimerism persisted. All NSGF8KO recipients (n = 7) that received 2bF8LV-transduced hCB cells survived tail clipping if animals had greater than 2% of platelets derived from 2bF8LV-transduced hCB cells, whereas 5 of 7 survived when human platelets were 0.3% to 2%. Whole blood clotting time analysis confirmed that hemostasis was improved in NSGF8KO mice that received 2bF8LV-transduced hCB cells. We demonstrate, for the first time, the feasibility of 2bF8LV gene delivery to human hematopoietic stem cells to introduce FVIII expression in human platelets and that human platelet-derived FVIII can improve hemostasis in hemophilia A.
Figures



References
-
- Hay CR, Brown S, Collins PW, Keeling DM, Liesner R. The diagnosis and management of factor VIII and IX inhibitors: a guideline from the United Kingdom Haemophilia Centre Doctors Organisation. Br J Haematol. 2006;133(6):591–605. - PubMed
-
- Gouw SC, van den Berg HM, le Cessie S, van der Bom JG. Treatment characteristics and the risk of inhibitor development: a multicenter cohort study among previously untreated patients with severe hemophilia A. J Thromb Haemost. 2007;5(7):1383–1390. - PubMed
-
- Wong T, Recht M. Current options and new developments in the treatment of haemophilia. Drugs. 2011;71(3):305–320. - PubMed
-
- Gouw SC, van der Bom JG, Marijke van den Berg H. Treatment-related risk factors of inhibitor development in previously untreated patients with hemophilia A: the CANAL cohort study. Blood. 2007;109(11):4648–4654. - PubMed
-
- Ingerslev J, Sørensen B. Parallel use of by-passing agents in haemophilia with inhibitors: a critical review. Br J Haematol. 2011;155(2):256–262. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

