Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology
- PMID: 24270007
- PMCID: PMC4219254
- DOI: 10.1016/j.addr.2013.11.009
Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology
Abstract
Cancer nanotherapeutics are progressing at a steady rate; research and development in the field has experienced an exponential growth since early 2000's. The path to the commercialization of oncology drugs is long and carries significant risk; however, there is considerable excitement that nanoparticle technologies may contribute to the success of cancer drug development. The pace at which pharmaceutical companies have formed partnerships to use proprietary nanoparticle technologies has considerably accelerated. It is now recognized that by enhancing the efficacy and/or tolerability of new drug candidates, nanotechnology can meaningfully contribute to create differentiated products and improve clinical outcome. This review describes the lessons learned since the commercialization of the first-generation nanomedicines including DOXIL® and Abraxane®. It explores our current understanding of targeted and non-targeted nanoparticles that are under various stages of development, including BIND-014 and MM-398. It highlights the opportunities and challenges faced by nanomedicines in contemporary oncology, where personalized medicine is increasingly the mainstay of cancer therapy. We revisit the fundamental concepts of enhanced permeability and retention effect (EPR) and explore the mechanisms proposed to enhance preferential "retention" in the tumor, whether using active targeting of nanoparticles, binding of drugs to their tumoral targets or the presence of tumor associated macrophages. The overall objective of this review is to enhance our understanding in the design and development of therapeutic nanoparticles for treatment of cancers.
Keywords: Active targeting; Drug delivery; Enhanced permeation and retention effect; Imaging; Nanomedicine; Nanoparticles; Patient enrichment; Personalized medicine; Tumor microenvironment; Vessel normalization.
Copyright © 2013 Elsevier B.V. All rights reserved.
Conflict of interest statement
The rest of the authors declare no conflict of interest.
Figures

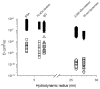

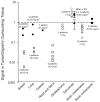


References
-
- Strebhardt K, Ullrich A. Paul Ehrlich’s magical bullet concept: 100 years of progress. Nat Rev Cancer. 2008;8:473–480. - PubMed
-
- Tabernero J, Shapiro GI, LoRusso PM, Cervantes A, Schwartz GK, Weiss GJ, Paz-Ares L, Cho DC, Infante JR, Alsina M, Gounder MM, Falzone R, Harrop J, White ACS, Toudjarska I, Bumcrot D, Meyers RE, Hinkle G, Svrzikapa N, Hutabarat RM, Clausen VA, Cehelsky J, Nochur SV, Gamba-Vitalo C, Vaishnaw AK, Sah DWY, Gollob JA, Burris HA. First-in-Humans Trial of an RNA Interference Therapeutic Targeting VEGF and KSP in Cancer Patients with Liver Involvement. Cancer Discovery. 2013;3:406–417. - PubMed
-
- Hrkach J, Von Hoff D, Ali MM, Andrianova E, Auer J, Campbell T, De Witt D, Figa M, Figueiredo M, Horhota A, Low S, McDonnell K, Peeke E, Retnajaran B, Sabnis A, Schnipper E, Song JJ, Song YH, Summa J, Tompsett D, Troiano G, Van Geen Hoven T, Wright J, LoRusso P, Kantoff PW, Bander NH, Sweeney C, Farokhzad OC, Langer R, Zale S. Preclinical development and clinical translation of a PSMA-targeted docetaxel nanoparticle with a differenciated pharmacological profile. Sci Transl Med. 2012;4:128ra139. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

