Nanopreparations for organelle-specific delivery in cancer
- PMID: 24270008
- PMCID: PMC3947805
- DOI: 10.1016/j.addr.2013.11.004
Nanopreparations for organelle-specific delivery in cancer
Abstract
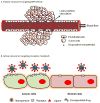
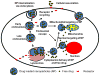
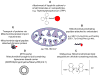
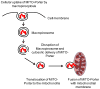
To efficiently deliver therapeutics into cancer cells, a number of strategies have been recently investigated. The toxicity associated with the administration of chemotherapeutic drugs due to their random interactions throughout the body necessitates the development of drug-encapsulating nanopreparations that significantly mask, or reduce, the toxic side effects of the drugs. In addition to reduced side effects associated with drug encapsulation, nanocarriers preferentially accumulate in tumors as a result of its abnormally leaky vasculature via the Enhanced Permeability and Retention (EPR) effect. However, simple passive nanocarrier delivery to the tumor site is unlikely to be enough to elicit a maximum therapeutic response as the drug-loaded carriers must reach the intracellular target sites. Therefore, efficient translocation of the nanocarrier through the cell membrane is necessary for cytosolic delivery of the cargo. However, crossing the cell membrane barrier and reaching cytosol might still not be enough for achieving maximum therapeutic benefit, which necessitates the delivery of drugs directly to intracellular targets, such as bringing pro-apoptotic drugs to mitochondria, nucleic acid therapeutics to nuclei, and lysosomal enzymes to defective lysosomes. In this review, we discuss the strategies developed for tumor targeting, cytosolic delivery via cell membrane translocation, and finally organelle-specific targeting, which may be applied for developing highly efficacious, truly multifunctional, cancer-targeted nanopreparations.
Keywords: Cancer; Drug delivery; Endocytosis; Intracellular; Nanopreparations; Organelle-specific.
Copyright © 2013 Elsevier B.V. All rights reserved.
Figures


References
-
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. - PubMed
-
- Alexis F, Pridgen EM, Langer R, Farokhzad OC. Nanoparticle technologies for cancer therapy. Handb Exp Pharmacol. 2010:55–86. - PubMed
-
- Tiwari M. Nano cancer therapy strategies. J Cancer Res Ther. 2012;8:19–22. - PubMed
-
- Langer R. Drug delivery and targeting. Nature. 1998;392:5–10. - PubMed
-
- Farokhzad OC, Langer R. Impact of nanotechnology on drug delivery. ACS Nano. 2009;3:16–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

