Mammalian target of rapamycin complex 2 (mTORC2) coordinates pulmonary artery smooth muscle cell metabolism, proliferation, and survival in pulmonary arterial hypertension
- PMID: 24270265
- PMCID: PMC3968690
- DOI: 10.1161/CIRCULATIONAHA.113.004581
Mammalian target of rapamycin complex 2 (mTORC2) coordinates pulmonary artery smooth muscle cell metabolism, proliferation, and survival in pulmonary arterial hypertension
Abstract
Background: Enhanced proliferation, resistance to apoptosis, and metabolic shift to glycolysis of pulmonary arterial vascular smooth muscle cells (PAVSMCs) are key pathophysiological components of pulmonary vascular remodeling in idiopathic pulmonary arterial hypertension (PAH). The role of the distinct mammalian target of rapamycin (mTOR) complexes mTORC1 (mTOR-Raptor) and mTORC2 (mTOR-Rictor) in PAVSMC proliferation and survival in PAH and their therapeutic relevance are unknown.
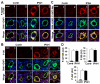
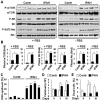
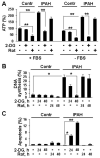
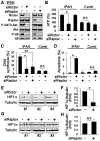
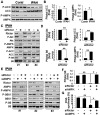
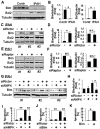
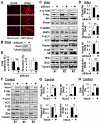
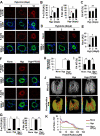
Methods and results: Immunohistochemical and immunoblot analyses revealed that mTORC1 and mTORC2 pathways are markedly upregulated in small remodeled pulmonary arteries and isolated distal PAVSMCs from subjects with idiopathic PAH that have increased ATP levels, proliferation, and survival that depend on glycolytic metabolism. Small interfering RNA- and pharmacology-based analysis showed that although both mTORC1 and mTORC2 contribute to proliferation, only mTORC2 is required for ATP generation and survival of idiopathic PAH PAVSMCs. mTORC2 downregulated the energy sensor AMP-activated protein kinase, which led to activation of mTORC1-S6 and increased proliferation, as well as a deficiency of the proapoptotic protein Bim and idiopathic PAH PAVSMC survival. NADPH oxidase 4 (Nox4) protein levels were increased in idiopathic PAH PAVSMCs, which was necessary for mTORC2 activation, proliferation, and survival. Nox4 levels and mTORC2 signaling were significantly upregulated in small pulmonary arteries from hypoxia-exposed rats at days 2 to 28 of hypoxia. Treatment with the mTOR kinase inhibitor PP242 at days 15 to 28 suppressed mTORC2 but not Nox4, induced smooth muscle-specific apoptosis in small pulmonary arteries, and reversed hypoxia-induced pulmonary vascular remodeling in rats.
Conclusions: These data provide a novel mechanistic link of Nox4-dependent activation of mTORC2 via the energy sensor AMP-activated protein kinase to increased proliferation and survival of PAVSMCs in PAH, which suggests a new potential pathway for therapeutic interventions.
Keywords: AMP-activated protein kinase; energy metabolism; idiopathic pulmonary arterial hypertension; mTORC2; muscle, smooth, vascular; remodeling; signal transduction.
Figures
Similar articles
-
Pharmacological Inhibition of mTOR Kinase Reverses Right Ventricle Remodeling and Improves Right Ventricle Structure and Function in Rats.Am J Respir Cell Mol Biol. 2017 Nov;57(5):615-625. doi: 10.1165/rcmb.2016-0364OC. Am J Respir Cell Mol Biol. 2017. PMID: 28679058 Free PMC article.
-
Up-regulation of the mammalian target of rapamycin complex 1 subunit Raptor by aldosterone induces abnormal pulmonary artery smooth muscle cell survival patterns to promote pulmonary arterial hypertension.FASEB J. 2016 Jul;30(7):2511-27. doi: 10.1096/fj.201500042. Epub 2016 Mar 22. FASEB J. 2016. PMID: 27006450 Free PMC article.
-
mTOR is required for pulmonary arterial vascular smooth muscle cell proliferation under chronic hypoxia.FASEB J. 2011 Jun;25(6):1922-33. doi: 10.1096/fj.10-175018. Epub 2011 Mar 2. FASEB J. 2011. PMID: 21368105 Free PMC article.
-
Targeted Inhibition of Rictor/mTORC2 in Cancer Treatment: A New Era after Rapamycin.Curr Cancer Drug Targets. 2016;16(4):288-304. doi: 10.2174/1568009616666151113120830. Curr Cancer Drug Targets. 2016. PMID: 26563881 Review.
-
mTOR and vascular remodeling in lung diseases: current challenges and therapeutic prospects.FASEB J. 2013 May;27(5):1796-807. doi: 10.1096/fj.12-222224. Epub 2013 Jan 25. FASEB J. 2013. PMID: 23355268 Free PMC article. Review.
Cited by
-
Inhibitory Antibodies against Activin A and TGF-β Reduce Self-Supported, but Not Soluble Factors-Induced Growth of Human Pulmonary Arterial Vascular Smooth Muscle Cells in Pulmonary Arterial Hypertension.Int J Mol Sci. 2018 Sep 28;19(10):2957. doi: 10.3390/ijms19102957. Int J Mol Sci. 2018. PMID: 30274147 Free PMC article.
-
Cellular Pathways Promoting Pulmonary Vascular Remodeling by Hypoxia.Physiology (Bethesda). 2020 Jul 1;35(4):222-233. doi: 10.1152/physiol.00039.2019. Physiology (Bethesda). 2020. PMID: 32490752 Free PMC article. Review.
-
Loss of Rictor in Monocyte/Macrophages Suppresses Their Proliferation and Viability Reducing Atherosclerosis in LDLR Null Mice.Front Immunol. 2018 Feb 13;9:215. doi: 10.3389/fimmu.2018.00215. eCollection 2018. Front Immunol. 2018. PMID: 29487597 Free PMC article.
-
Regulation of Smooth Muscle Cell Proliferation by NADPH Oxidases in Pulmonary Hypertension.Antioxidants (Basel). 2019 Mar 5;8(3):56. doi: 10.3390/antiox8030056. Antioxidants (Basel). 2019. PMID: 30841544 Free PMC article. Review.
-
The role of inflammation in hypoxic pulmonary hypertension: from cellular mechanisms to clinical phenotypes.Am J Physiol Lung Cell Mol Physiol. 2015 Feb 1;308(3):L229-52. doi: 10.1152/ajplung.00238.2014. Epub 2014 Nov 21. Am J Physiol Lung Cell Mol Physiol. 2015. PMID: 25416383 Free PMC article. Review.
References
-
- Archer SL, Gomberg-Maitland M, Maitland ML, Rich S, Garcia JGN, Weir EK. Mitochondrial metabolism, redox signaling, and fusion: a mitochondria-ROS-HIF-1α-Kv1.5 O2-sensing pathway at the intersection of pulmonary hypertension and cancer. Am J Physiol Heart Circ Physiol. 2008;294:H570–H578. - PubMed
-
- Masri FA, Xu W, Comhair SAA, Asosingh K, Koo M, Vasanji A, Drazba J, Anand-Apte B, Erzurum SC. Hyperproliferative apoptosis-resistant endothelial cells in idiopathic pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol. 2007;293:L548–L554. - PubMed
-
- Das M, Dempsey EC, Bouchey D, Reyland ME, Stenmark KR. Chronic Hypoxia Induces Exaggerated Growth Responses in Pulmonary Artery Adventitial Fibroblasts. Potential Contribution of Specific Protein Kinase C Isozymes. Am J Respir Cell Mol Biol. 2000;22:15–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

