Mice generated by in vitro fertilization exhibit vascular dysfunction and shortened life span
- PMID: 24270419
- PMCID: PMC3859389
- DOI: 10.1172/JCI68943
Mice generated by in vitro fertilization exhibit vascular dysfunction and shortened life span
Abstract
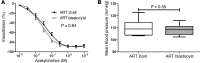
Children conceived by assisted reproductive technologies (ART) display a level of vascular dysfunction similar to that seen in children of mothers with preeclamspia. The long-term consequences of ART-associated vascular disorders are unknown and difficult to investigate in healthy children. Here, we found that vasculature from mice generated by ART display endothelial dysfunction and increased stiffness, which translated into arterial hypertension in vivo. Progeny of male ART mice also exhibited vascular dysfunction, suggesting underlying epigenetic modifications. ART mice had altered methylation at the promoter of the gene encoding eNOS in the aorta, which correlated with decreased vascular eNOS expression and NO synthesis. Administration of a deacetylase inhibitor to ART mice normalized vascular gene methylation and function and resulted in progeny without vascular dysfunction. The induction of ART-associated vascular and epigenetic alterations appeared to be related to the embryo environment; these alterations were possibly facilitated by the hormonally stimulated ovulation accompanying ART. Finally, ART mice challenged with a high-fat diet had roughly a 25% shorter life span compared with control animals. This study highlights the potential of ART to induce vascular dysfunction and shorten life span and suggests that epigenetic alterations contribute to these problems.
Figures







References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

