Perivascular macrophages mediate neutrophil recruitment during bacterial skin infection
- PMID: 24270515
- PMCID: PMC4097073
- DOI: 10.1038/ni.2769
Perivascular macrophages mediate neutrophil recruitment during bacterial skin infection
Abstract
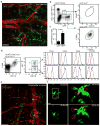
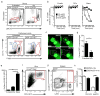
Transendothelial migration of neutrophils in postcapillary venules is a key event in the inflammatory response against pathogens and tissue damage. The precise regulation of this process is incompletely understood. We report that perivascular macrophages are critical for neutrophil migration into skin infected with the pathogen Staphylococcus aureus. Using multiphoton intravital microscopy we showed that neutrophils extravasate from inflamed dermal venules in close proximity to perivascular macrophages, which are a major source of neutrophil chemoattractants. The virulence factor α-hemolysin produced by S. aureus lyses perivascular macrophages, which leads to decreased neutrophil transmigration. Our data illustrate a previously unrecognized role for perivascular macrophages in neutrophil recruitment to inflamed skin and indicate that S. aureus uses hemolysin-dependent killing of these cells as an immune evasion strategy.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




Comment in
-
Neutrophil recruitment: perivascular macrophages 'duke it out' with Staphylococcus aureus.Nat Immunol. 2014 Jan;15(1):10-1. doi: 10.1038/ni.2793. Nat Immunol. 2014. PMID: 24352318 No abstract available.
References
-
- Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13:159–175. - PubMed
-
- Nourshargh S, Hordijk PL, Sixt M. Breaching multiple barriers: leukocyte motility through venular walls and the interstitium. Nat Rev Mol Cell Biol. 2010;11:366–378. - PubMed
-
- Kansas G. Selectins and their ligands: current concepts and controversies. Blood. 1996;88:3259–3287. - PubMed
-
- Laudanna C, Kim JY, Constantin G, Butcher E. Rapid leukocyte integrin activation by chemokines. Immunol Rev. 2002;186:37–46. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

