Somatic cells regulate maternal mRNA translation and developmental competence of mouse oocytes
- PMID: 24270888
- PMCID: PMC4066669
- DOI: 10.1038/ncb2873
Somatic cells regulate maternal mRNA translation and developmental competence of mouse oocytes
Abstract
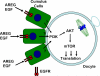
Germ cells divide and differentiate in a unique local microenvironment under the control of somatic cells. Signals released in this niche instruct oocyte reentry into the meiotic cell cycle. Once initiated, the progression through meiosis and the associated programme of maternal messenger RNA translation are thought to be cell autonomous. Here we show that translation of a subset of maternal mRNAs critical for embryo development is under the control of somatic cell inputs. Translation of specific maternal transcripts increases in oocytes cultured in association with somatic cells and is sensitive to EGF-like growth factors that act only on the somatic compartment. In mice deficient in amphiregulin, decreased fecundity and oocyte developmental competence is associated with defective translation of a subset of maternal mRNAs. These somatic cell signals that affect translation require activation of the PI(3)K-AKT-mTOR pathway. Thus, mRNA translation depends on somatic cell cues that are essential to reprogramme the oocyte for embryo development.
Figures
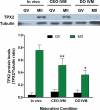
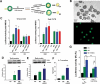
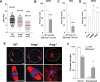
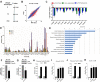

Comment in
-
Somatic guidance for the oocyte.Dev Cell. 2013 Dec 23;27(6):603-5. doi: 10.1016/j.devcel.2013.12.006. Dev Cell. 2013. PMID: 24369833
References
-
- Yoshida S. Spermatogenic stem cell system in the mouse testis. Cold Spring Harb Symp Quant Biol. 2008;73:25–32. - PubMed
-
- Matzuk MM, Burns KH, Viveiros MM, Eppig JJ. Intercellular communication in the mammalian ovary: oocytes carry the conversation. Science. 2002;296:2178–2180. - PubMed
-
- Chian RC, Lim JH, Tan SL. State of the art in in-vitro oocyte maturation. Curr Opin Obstet Gynecol. 2004;16:211–219. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

