Beyond the zebrafish: diverse fish species for modeling human disease
- PMID: 24271780
- PMCID: PMC3917239
- DOI: 10.1242/dmm.012245
Beyond the zebrafish: diverse fish species for modeling human disease
Abstract
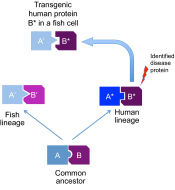
In recent years, zebrafish, and to a lesser extent medaka, have become widely used small animal models for human diseases. These organisms have convincingly demonstrated the usefulness of fish for improving our understanding of the molecular and cellular mechanisms leading to pathological conditions, and for the development of new diagnostic and therapeutic tools. Despite the usefulness of zebrafish and medaka in the investigation of a wide spectrum of traits, there is evidence to suggest that other fish species could be better suited for more targeted questions. With the emergence of new, improved sequencing technologies that enable genomic resources to be generated with increasing efficiency and speed, the potential of non-mainstream fish species as disease models can now be explored. A key feature of these fish species is that the pathological condition that they model is often related to specific evolutionary adaptations. By exploring these adaptations, new disease-causing and disease-modifier genes might be identified; thus, diverse fish species could be exploited to better understand the complexity of disease processes. In addition, non-mainstream fish models could allow us to study the impact of environmental factors, as well as genetic variation, on complex disease phenotypes. This Review will discuss the opportunities that such fish models offer for current and future biomedical research.
Keywords: Cancer; Evolutionary mutant model; Fish model; Natural variation.
Figures






References
-
- Albertson R. C., Kocher T. D. (2006). Genetic and developmental basis of cichlid trophic diversity. Heredity 97, 211–221 - PubMed
-
- Amatruda J. F., Patton E. E. (2008). Genetic models of cancer in zebrafish. Int Rev Cell Mol Biol 271, 1–34 - PubMed
-
- Amores A., Force A., Yan Y. L., Joly L., Amemiya C., Fritz A., Ho R. K., Langeland J., Prince V., Wang Y. L., et al. (1998). Zebrafish hox clusters and vertebrate genome evolution. Science 282, 1711–1714 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

