Blue light-dependent interaction between cryptochrome2 and CIB1 regulates transcription and leaf senescence in soybean
- PMID: 24272488
- PMCID: PMC3875726
- DOI: 10.1105/tpc.113.116590
Blue light-dependent interaction between cryptochrome2 and CIB1 regulates transcription and leaf senescence in soybean
Abstract
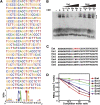
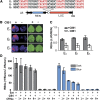
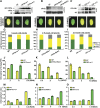
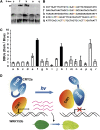
Cryptochromes are blue light receptors that regulate light responses in plants, including various crops. The molecular mechanism of plant cryptochromes has been extensively investigated in Arabidopsis thaliana, but it has not been reported in any crop species. Here, we report a study of the mechanism of soybean (Glycine max) cryptochrome2 (CRY2a). We found that CRY2a regulates leaf senescence, which is a life history trait regulated by light and photoperiods via previously unknown mechanisms. We show that CRY2a undergoes blue light-dependent interaction with the soybean basic helix-loop-helix transcription activator CIB1 (for cryptochrome-interacting bHLH1) that specifically interacts with the E-box (CANNTG) DNA sequences. Analyses of transgenic soybean plants expressing an elevated or reduced level of the CRY2a or CIB1 demonstrate that CIB1 promotes leaf senescence, whereas CRY2a suppresses leaf senescence. Results of the gene expression and molecular interaction analyses support the hypothesis that CIB1 activates transcription of senescence-associated genes, such as WRKY DNA BINDING PROTEIN53b (WRKY53b), and leaf senescence. CIB1 interacts with the E-box-containing promoter sequences of the WRKY53b chromatin, whereas photoexcited CRY2a interacts with CIB1 to inhibit its DNA binding activity. These findings argue that CIB-dependent transcriptional regulation is an evolutionarily conserved CRY-signaling mechanism in plants, and this mechanism is opted in evolution to mediate light regulation of different aspects of plant development in different plant species.
Figures




References
-
- Ahmad M., Cashmore A.R. (1993). HY4 gene of A. thaliana encodes a protein with characteristics of a blue-light photoreceptor. Nature 366: 162–166 - PubMed
-
- Cashmore A.R. (2003). Cryptochromes: Enabling plants and animals to determine circadian time. Cell 114: 537–543 - PubMed
-
- Chaves I., Pokorny R., Byrdin M., Hoang N., Ritz T., Brettel K., Essen L.O., van der Horst G.T., Batschauer A., Ahmad M. (2011). The cryptochromes: Blue light photoreceptors in plants and animals. Annu. Rev. Plant Biol. 62: 335–364 - PubMed
-
- Griffin E.A., Jr, Staknis D., Weitz C.J. (1999). Light-independent role of CRY1 and CRY2 in the mammalian circadian clock. Science 286: 768–771 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

