An early evaluation of clinical and economic costs and benefits of implementing point of care NAAT tests for Chlamydia trachomatis and Neisseria gonorrhoea in genitourinary medicine clinics in England
- PMID: 24273127
- PMCID: PMC3932743
- DOI: 10.1136/sextrans-2013-051147
An early evaluation of clinical and economic costs and benefits of implementing point of care NAAT tests for Chlamydia trachomatis and Neisseria gonorrhoea in genitourinary medicine clinics in England
Abstract
Objectives: To estimate the costs and benefits of clinical pathways incorporating a point of care (POC) nucleic acid amplification test (NAAT) for chlamydia and gonorrhoea in genitourinary medicine (GUM) clinics compared with standard off-site laboratory testing.
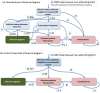
Method: We simulated 1.2 million GUM clinic attendees in England. A simulation in Microsoft Excel was developed to compare existing standard pathways of management for chlamydia and gonorrhoea with a POC NAAT. We conducted scenario analyses to evaluate the robustness of the model findings. The primary outcome was the incremental cost-effectiveness ratio. Secondary outcomes included the number of inappropriate treatments, complications and transmissions averted.
Results: The baseline cost of using the point of POC NAAT was £103.9 million compared with £115.6 million for standard care. The POC NAAT was also associated with a small increase of 46 quality adjusted life years, making the new test both more effective and cheaper. Over 95 000 inappropriate treatments might be avoided by using a POC NAAT. Patients receive diagnosis and treatment on the same day as testing, which may also prevent 189 cases of pelvic inflammatory disease and 17 561 onward transmissions annually.
Discussion: Replacing standard laboratory tests for chlamydia and gonorrhoea with a POC test could be cost saving and patients would benefit from more accurate diagnosis and less unnecessary treatment. Overtreatment currently accounts for about a tenth of the reported treatments for chlamydia and gonorrhoea and POC NAATs would effectively eliminate the need for presumptive treatment.
Keywords: Chlamydia Trachomatis; Cost-Effectiveness; Diagnosis; Gonorrhoea; Mathematical Model.
Figures
Comment in
-
Is nucleic acid amplification point-of-care testing for chlamydia and gonorrhoea cost-effective?Sex Transm Infect. 2014 Mar;90(2):82. doi: 10.1136/sextrans-2013-051428. Epub 2014 Jan 15. Sex Transm Infect. 2014. PMID: 24431187 No abstract available.
References
-
- Health Protection Agency. 2012. STI Data Tables. http://www.hpa.org.uk.
-
- Horner PJ, Boag FC. 2006 UK National Guideline for the Management of Genital Tract Infection with Chlamydia trachomatis. British Association of Sexual Health & HIV, 2006
-
- Bignell CJ, FitzGerald M. UK national guideline for the management of gonorrhoea in adults, 2011. Int J STI AIDS 2011;22:551–47 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
