Latrophilins function as heterophilic cell-adhesion molecules by binding to teneurins: regulation by alternative splicing
- PMID: 24273166
- PMCID: PMC3879561
- DOI: 10.1074/jbc.M113.504779
Latrophilins function as heterophilic cell-adhesion molecules by binding to teneurins: regulation by alternative splicing
Abstract
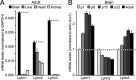
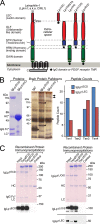
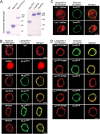
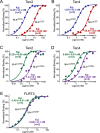
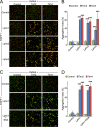
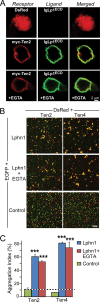
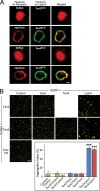
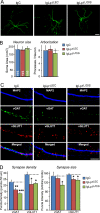
Latrophilin-1, -2, and -3 are adhesion-type G protein-coupled receptors that are auxiliary α-latrotoxin receptors, suggesting that they may have a synaptic function. Using pulldowns, we here identify teneurins, type II transmembrane proteins that are also candidate synaptic cell-adhesion molecules, as interactors for the lectin-like domain of latrophilins. We show that teneurin binds to latrophilins with nanomolar affinity and that this binding mediates cell adhesion, consistent with a role of teneurin binding to latrophilins in trans-synaptic interactions. All latrophilins are subject to alternative splicing at an N-terminal site; in latrophilin-1, this alternative splicing modulates teneurin binding but has no effect on binding of latrophilin-1 to another ligand, FLRT3. Addition to cultured neurons of soluble teneurin-binding fragments of latrophilin-1 decreased synapse density, suggesting that latrophilin binding to teneurin may directly or indirectly influence synapse formation and/or maintenance. These observations are potentially intriguing in view of the proposed role for Drosophila teneurins in determining synapse specificity. However, teneurins in Drosophila were suggested to act as homophilic cell-adhesion molecules, whereas our findings suggest a heterophilic interaction mechanism. Thus, we tested whether mammalian teneurins also are homophilic cell-adhesion molecules, in addition to binding to latrophilins as heterophilic cell-adhesion molecules. Strikingly, we find that although teneurins bind to each other in solution, homophilic teneurin-teneurin binding is unable to support stable cell adhesion, different from heterophilic teneurin-latrophilin binding. Thus, mammalian teneurins act as heterophilic cell-adhesion molecules that may be involved in trans-neuronal interaction processes such as synapse formation or maintenance.
Keywords: Alternative Splicing; Cell Adhesion; FLRT3; G Protein-coupled Receptors (GPCR); Ligand-binding Protein; Neurexin; Neurons; Synapse Formation; Teneurin; α-Latrotoxin.
Figures

References
-
- Krasnoperov V. G., Bittner M. A., Beavis R., Kuang Y., Salnikow K. V., Chepurny O. G., Little A. R., Plotnikov A. N., Wu D., Holz R. W., Petrenko A. G. (1997) α-Latrotoxin stimulates exocytosis by the interaction with a neuronal G-protein-coupled receptor. Neuron 18, 925–937 - PubMed
-
- Lelianova V. G., Davletov B. A., Sterling A., Rahman M. A., Grishin E. V., Totty N. F., Ushkaryov Y. A. (1997) α-Latrotoxin receptor, latrophilin, is a novel member of the secretin family of G protein-coupled receptors. J. Biol. Chem. 272, 21504–21508 - PubMed
-
- Levine A., Bashan-Ahrend A., Budai-Hadrian O., Gartenberg D., Menasherow S., Wides R. (1994) Odd Oz: a novel Drosophila pair rule gene. Cell 77, 587–598 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

