NLRP3 inflammasome contributes to inflammation after intracerebral hemorrhage
- PMID: 24273204
- PMCID: PMC4386653
- DOI: 10.1002/ana.24070
NLRP3 inflammasome contributes to inflammation after intracerebral hemorrhage
Abstract
Objective: The NLRP3 (NALP3, cryopyrin) inflammasome, a key component of the innate immune system, facilitates caspase-1 and interleukin (IL)-1β processing, which amplifies the inflammatory response. Here, we investigated whether NLRP3 knockdown decreases neutrophil infiltration, reduces brain edema, and improves neurological function in an intracerebral hemorrhage (ICH) mouse model. We also determined whether mitochondrial reactive oxygen species (ROS) governed by mitochondrial permeability transition pores (mPTPs) would trigger NLRP3 inflammasome activation following ICH.
Methods: ICH was induced by injecting autologous arterial blood (30μl) into a mouse brain. NLRP3 small interfering RNAs were administered 24 hours before ICH. A mPTP inhibitor (TRO-19622) or a specific mitochondria ROS scavenger (Mito-TEMPO) was coinjected with the blood. In naive animals, rotenone, which is a respiration chain complex I inhibitor, was applied to induce mitochondrial ROS production, and followed by TRO-19622 or Mito-TEMPO treatment. Neurological deficits, brain edema, enzyme-linked immunosorbent assay, Western blot, in vivo chemical cross-linking, ROS assay, and immunofluorescence were evaluated.
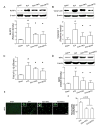
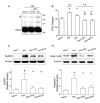
Results: ICH activated the NLRP3 inflammasome. NLRP3 knockdown reduced brain edema and decreased myeloperoxidase (MPO) levels at 24 hours, and improved neurological functions from 24 to 72 hours following ICH. TRO-19622 or Mito-TEMPO reduced ROS, NLRP3 inflammasome components, and MPO levels following ICH. In naive animals, rotenone administration induced mPTP formation, ROS generation, and NLRP3 inflammasome activation, which were then reduced by TRO-19622 or Mito-TEMPO.
Interpretation: The NLRP3 inflammasome amplified the inflammatory response by releasing IL-1β and promoting neutrophil infiltration following ICH. Mitochondria ROS may be a major trigger of NLRP3 inflammasome activation. The results of our study suggest that the inhibition of the NLRP3 inflammasome may effectively reduce the inflammatory response following ICH.ANN NEUROL 2014;75:209-219.
© 2013 Child Neurology Society/American Neurological Association.
Conflict of interest statement
Figures




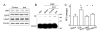
Comment in
-
Reactive oxygen species and NLRP3 inflammasome activation.Ann Neurol. 2014 Jun;75(6):972. doi: 10.1002/ana.24173. Epub 2014 May 28. Ann Neurol. 2014. PMID: 24798299 No abstract available.
-
Reply: To PMID 24273204.Ann Neurol. 2014 Jun;75(6):972-973. doi: 10.1002/ana.24175. Epub 2014 May 28. Ann Neurol. 2014. PMID: 24805252 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

