Advanced Motion Compensation Methods for Intravital Optical Microscopy
- PMID: 24273405
- PMCID: PMC3832946
- DOI: 10.1109/JSTQE.2013.2279314
Advanced Motion Compensation Methods for Intravital Optical Microscopy
Abstract
Intravital microscopy has emerged in the recent decade as an indispensible imaging modality for the study of the micro-dynamics of biological processes in live animals. Technical advancements in imaging techniques and hardware components, combined with the development of novel targeted probes and new mice models, have enabled us to address long-standing questions in several biology areas such as oncology, cell biology, immunology and neuroscience. As the instrument resolution has increased, physiological motion activities have become a major obstacle that prevents imaging live animals at resolutions analogue to the ones obtained in vitro. Motion compensation techniques aim at reducing this gap and can effectively increase the in vivo resolution. This paper provides a technical review of some of the latest developments in motion compensation methods, providing organ specific solutions.
Keywords: Intravital microscopy; image stabilization; in vivo imaging; motion artifact and motion compensation.
Figures
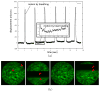
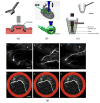

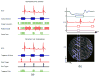



References
-
- Bousso P, Moreau HD. Functional immunoimaging: the revolution continues. Nat Rev Immunol. 2012 Dec;12:858–64. - PubMed
-
- Ritsma L, Ponsioen B, van Rheenen J. Intravital imaging of cell signaling in mice. IntraVital. 2012;1:0–8.
-
- Mai W, Badea CT, Wheeler CT, Hedlund LW, Johnson GA. Effects of breathing and cardiac motion on spatial resolution in the microscopic imaging of rodents. Magn Reson Med. 2005 Apr;53:858–65. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
