GABA and Glutamate Transporters in Brain
- PMID: 24273530
- PMCID: PMC3822327
- DOI: 10.3389/fendo.2013.00165
GABA and Glutamate Transporters in Brain
Abstract
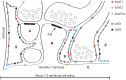
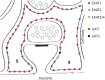
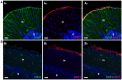
The mammalian genome contains four genes encoding GABA transporters (GAT1, slc6a1; GAT2, slc6a13; GAT3, slc6a11; BGT1, slc6a12) and five glutamate transporter genes (EAAT1, slc1a3; EAAT2, slc1a2; EAAT3, slc1a1; EAAT4, slc1a6; EAAT5, slc1a7). These transporters keep the extracellular levels of GABA and excitatory amino acids low and provide amino acids for metabolic purposes. The various transporters have different properties both with respect to their transport functions and with respect to their ability to act as ion channels. Further, they are differentially regulated. To understand the physiological roles of the individual transporter subtypes, it is necessary to obtain information on their distributions and expression levels. Quantitative data are important as the functional capacity is limited by the number of transporter molecules. The most important and most abundant transporters for removal of transmitter glutamate in the brain are EAAT2 (GLT-1) and EAAT1 (GLAST), while GAT1 and GAT3 are the major GABA transporters in the brain. EAAT3 (EAAC1) does not appear to play a role in signal transduction, but plays other roles. Due to their high uncoupled anion conductance, EAAT4 and EAAT5 seem to be acting more like inhibitory glutamate receptors than as glutamate transporters. GAT2 and BGT1 are primarily expressed in the liver and kidney, but are also found in the leptomeninges, while the levels in brain tissue proper are too low to have any impact on GABA removal, at least in normal young adult mice. The present review will provide summary of what is currently known and will also discuss some methodological pitfalls.
Keywords: BGT1; EAAT1; EAAT2; GABA uptake; GAT1; GAT2; GAT3; glutamate uptake.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

