Structure and stereospecificity of the dehydratase domain from the terminal module of the rifamycin polyketide synthase
- PMID: 24274103
- PMCID: PMC3948214
- DOI: 10.1021/bi400988t
Structure and stereospecificity of the dehydratase domain from the terminal module of the rifamycin polyketide synthase
Abstract
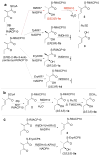
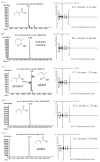
RifDH10, the dehydratase domain from the terminal module of the rifamycin polyketide synthase, catalyzes the stereospecific syn dehydration of the model substrate (2S,3S)-2-methyl-3-hydroxypentanoyl-RifACP10, resulting in the exclusive formation of (E)-2-methyl-2-pentenoyl-RifACP10. RifDH10 does not dehydrate any of the other three diastereomeric, RifACP10-bound, diketide thioester substrates. On the other hand, when EryACP6, from the sixth module of the erythromycin polyketide synthase, is substituted for RifACP10, RifDH10 stereospecifically dehydrates only (2R,3R)-2-methyl-3-hydroxypentanoyl-EryACP6 to give exclusively (E)-2-methyl-2-pentenoyl-EryACP6, with no detectable dehydration of any of the other three diastereomeric, EryACP6-bound, diketides. An identical alteration in substrate diastereospecificity was observed for the corresponding N-acetylcysteamine or pantetheine thioester analogues, regardless of acyl chain length or substitution pattern. Incubation of (2RS)-2-methyl-3-ketopentanoyl-RifACP10 with the didomain reductase-dehydratase RifKR10-RifDH10 yielded (E)-2-methyl-2-pentenoyl-RifACP10, the expected product of syn dehydration of (2S,3S)-2-methyl-3-hydroxypentanoyl-RifACP10, while incubation with the corresponding EryACP6-bound substrate, (2RS)-2-methyl-3-ketopentanoyl-EryACP6, gave only the reduction product (2S,3S)-2-methyl-3-hydroxypentanoyl-EryACP6 with no detectable dehydration. These results establish the intrinsic syn dehydration stereochemistry and substrate diastereoselectivity of RifDH10 and highlight the critical role of the natural RifACP10 domain in chaperoning the proper recognition and processing of the natural ACP-bound undecaketide substrate. The 1.82 Å resolution structure of RifDH10 reveals the atomic-resolution details of the active site and allows modeling of the syn dehydration of the (2S,3S)-2-methyl-3-hydroxyacyl-RifACP10 substrate. These results suggest that generation of the characteristic cis double bond of the rifamycins occurs after formation of the full-length RifACP10-bound acyclic trans-unsaturated undecaketide intermediate, most likely during the subsequent macrolactamization catalyzed by the amide synthase RifF.
Figures





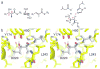
Similar articles
-
Stereospecificity of the dehydratase domain of the erythromycin polyketide synthase.J Am Chem Soc. 2010 Oct 27;132(42):14697-9. doi: 10.1021/ja107344h. J Am Chem Soc. 2010. PMID: 20925342 Free PMC article.
-
Mechanism and stereospecificity of a fully saturating polyketide synthase module: nanchangmycin synthase module 2 and its dehydratase domain.J Am Chem Soc. 2010 Oct 27;132(42):14694-6. doi: 10.1021/ja1073432. J Am Chem Soc. 2010. PMID: 20925339 Free PMC article.
-
Polyketide double bond biosynthesis. Mechanistic analysis of the dehydratase-containing module 2 of the picromycin/methymycin polyketide synthase.J Am Chem Soc. 2005 Dec 14;127(49):17393-404. doi: 10.1021/ja055672+. J Am Chem Soc. 2005. PMID: 16332089 Free PMC article.
-
Stereospecific Formation of Z-Trisubstituted Double Bonds by the Successive Action of Ketoreductase and Dehydratase Domains from trans-AT Polyketide Synthases.Biochemistry. 2018 Jun 5;57(22):3126-3129. doi: 10.1021/acs.biochem.7b01253. Epub 2018 Jan 5. Biochemistry. 2018. PMID: 29293329 Free PMC article.
-
Stereospecificity of ketoreductase domains 1 and 2 of the tylactone modular polyketide synthase.J Am Chem Soc. 2008 Sep 3;130(35):11598-9. doi: 10.1021/ja804453p. Epub 2008 Aug 12. J Am Chem Soc. 2008. PMID: 18693734 Free PMC article.
Cited by
-
Conservation of griseofulvin genes in the gsf gene cluster among fungal genomes.G3 (Bethesda). 2022 Feb 4;12(2):jkab399. doi: 10.1093/g3journal/jkab399. G3 (Bethesda). 2022. PMID: 34792561 Free PMC article.
-
Structural insights into dehydratase substrate selection for the borrelidin and fluvirucin polyketide synthases.J Ind Microbiol Biotechnol. 2019 Aug;46(8):1225-1235. doi: 10.1007/s10295-019-02189-z. Epub 2019 May 21. J Ind Microbiol Biotechnol. 2019. PMID: 31115703 Free PMC article.
-
Engineered polyketides: Synergy between protein and host level engineering.Synth Syst Biotechnol. 2017 Sep 7;2(3):147-166. doi: 10.1016/j.synbio.2017.08.005. eCollection 2017 Sep. Synth Syst Biotechnol. 2017. PMID: 29318196 Free PMC article. Review.
-
Tylosin polyketide synthase module 3: stereospecificity, stereoselectivity and steady-state kinetic analysis of β-processing domains via diffusible, synthetic substrates.Chem Sci. 2015 Aug 14;6(8):5027-5033. doi: 10.1039/c5sc01505g. Epub 2015 Jun 29. Chem Sci. 2015. PMID: 26366283 Free PMC article.
-
Polyketide stereocontrol: a study in chemical biology.Beilstein J Org Chem. 2017 Feb 24;13:348-371. doi: 10.3762/bjoc.13.39. eCollection 2017. Beilstein J Org Chem. 2017. PMID: 28326145 Free PMC article. Review.
References
-
- Sherman DH, Smith JL. Clearing the skies over modular polyketide synthases, ACS Chem. Biol. 2006;1:505–509. - PubMed
-
- Piel J. Biosynthesis of polyketides by trans-AT polyketide synthases, Nat. Prod Rep. 2010;27:996–1047. - PubMed
-
- Lewy DS, Gauss CM, Soenen DR, Boger DL. Fostriecin: chemistry and biology, Curr. Med Chem. 2002;9:2005–2032. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

