Molecular mechanisms for synchronous, asynchronous, and spontaneous neurotransmitter release
- PMID: 24274737
- PMCID: PMC4503208
- DOI: 10.1146/annurev-physiol-021113-170338
Molecular mechanisms for synchronous, asynchronous, and spontaneous neurotransmitter release
Abstract
Most neuronal communication relies upon the synchronous release of neurotransmitters, which occurs through synaptic vesicle exocytosis triggered by action potential invasion of a presynaptic bouton. However, neurotransmitters are also released asynchronously with a longer, variable delay following an action potential or spontaneously in the absence of action potentials. A compelling body of research has identified roles and mechanisms for synchronous release, but asynchronous release and spontaneous release are less well understood. In this review, we analyze how the mechanisms of the three release modes overlap and what molecular pathways underlie asynchronous and spontaneous release. We conclude that the modes of release have key fusion processes in common but may differ in the source of and necessity for Ca(2+) to trigger release and in the identity of the Ca(2+) sensor for release.
Figures



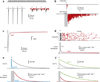
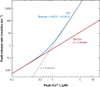

References
-
- Katz B, Miledi R. The measurement of synaptic delay, and the time course of acetylcholine release at the neuromuscular junction. Proc. R. Soc. Lond. B. 1965;161:483–495. - PubMed
-
- Borst JG, Sakmann B. Calcium influx and transmitter release in a fast CNS synapse. Nature. 1996;383:431–434. - PubMed
-
- Sabatini BL, Regehr WG. Timing of neurotransmission at fast synapses in the mammalian brain. Nature. 1996;384:170–172. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

