Chutes and ladders in hepatitis C nucleoside drug development
- PMID: 24275341
- PMCID: PMC3910353
- DOI: 10.1016/j.antiviral.2013.11.008
Chutes and ladders in hepatitis C nucleoside drug development
Abstract
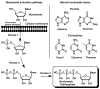
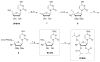
Chutes and Ladders is an exciting up-and-down-again game in which players race to be the first to the top of the board. Along the way, they will find ladders to help them advance, and chutes that will cause them to move backwards. The development of nucleoside analogs for clinical treatment of hepatitis C presents a similar scenario in which taking shortcuts may help quickly advance a program, but there is always a tremendous risk of being sent backwards as one competes for the finish line. In recent years the treatment options for chronic hepatitis C virus (HCV) infection have expand due to the development of a replicon based in vitro evaluation system, allowing for the identification of multiple drugable viral targets along with a concerted and substantial drug discovery effort. Three major drug targets have reached clinical study for chronic HCV infection: the NS3/4A serine protease, the large phosphoprotein NS5A, and the NS5B RNA-dependent RNA polymerase. Recently, two oral HCV protease inhibitors were approved by the FDA and were the first direct acting anti-HCV agents to result from the substantial research in this area. There are currently many new chemical entities from several different target classes that are being evaluated worldwide in clinical trials for their effectiveness at achieving a sustained virologic response (SVR) (Pham et al., 2004; Radkowski et al., 2005). Clearly the goal is to develop therapies leading to a cure that are safe, widely accessible and available, and effective against all HCV genotypes (GT), and all stages of the disease. Nucleoside analogs that target the HCV NS5B polymerase that have reached human clinical trials is the focus of this review as they have demonstrated significant advantages in the clinic with broader activity against the various HCV GT and a higher barrier to the development of resistant viruses when compared to all other classes of HCV inhibitors.
Keywords: Antiviral; Clinical trials; HCV; Nucleoside analog; Prodrug.
Published by Elsevier B.V.
Figures
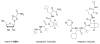
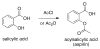
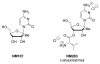
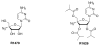
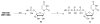



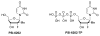
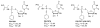
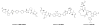



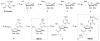
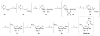
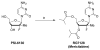








References
-
- Afdahl N, Rodriguez-Torres M, Lawitz E, Godofsky E, Chao G, Fielman B, Knox S, Brown N. Enhanced antiviral efficacy for valopicitabine (NM283) plus Peg-Interferon in hepatitis C patients with HCV genotype-1 infection: Results of a Phase IIa multicenter trial. J Hepatol. 2005;42:39–40.
-
- Ahmed HA, Alfredson TV, Birudaraj K, Brandl MT, Phuapradit W, Shah NH, Stefanidis D. HCV prodrug formulation. WO 2007068615 PCT Int Appl. 2007
-
- Alfredson T, Sarma K, Brandl M, Larrabee S, Wu X, Tu Y, Liu H, Smith D, Birudaraj R, Li F, Tran T, Hadig X, Hill G, Daley R, Blue D, Berger N, Symons J, Cammack N, Ipe D, Berns H, Lakatos I, Swallow S, Klumpp K. Design and evaluation of novel HCV polymerase inhibitor prodrugs with enhanced oral bioavailability. AAPS Annual Meeting; San Antonio, TX. 2006.
-
- Ali S, Leveque V, Le Pogam S, Ma H, Philipp F, Inocencio N, Smith M, Alker A, Kang H, Najera I, Klumpp K, Symons J, Cammack N, Jiang WL. Selected replicon variants with low-level in vitro resistance to the hepatitis C virus NS5B polymerase inhibitor PSI-6130 lack cross-resistance with R1479. Antimicrob Agents Chemother. 2008;52:4356–4369. - PMC - PubMed
-
- Arnold JJ, Sharma SD, Feng JY, Ray AS, Smidansky ED, Kireeva ML, Cho A, Perry J, Vela JE, Park Y, Xu Y, Tian Y, Babusis D, Barauskus O, Peterson BR, Gnatt A, Kashlev M, Zhong W, Cameron CE. Sensitivity of Mitochondrial Transcription and Resistance of RNA Polymerase II Dependant Nuclear Transcription to Antiviral Ribonucleosides. PLoS Pathog. 2012;8:e1003030. doi:1003010.1001371/journal.ppat.1003030. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

