Correlated cryo-fluorescence and cryo-electron microscopy with high spatial precision and improved sensitivity
- PMID: 24275379
- PMCID: PMC5472196
- DOI: 10.1016/j.ultramic.2013.10.015
Correlated cryo-fluorescence and cryo-electron microscopy with high spatial precision and improved sensitivity
Abstract
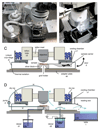
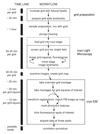
Performing fluorescence microscopy and electron microscopy on the same sample allows fluorescent signals to be used to identify and locate features of interest for subsequent imaging by electron microscopy. To carry out such correlative microscopy on vitrified samples appropriate for structural cryo-electron microscopy it is necessary to perform fluorescence microscopy at liquid-nitrogen temperatures. Here we describe an adaptation of a cryo-light microscopy stage to permit use of high-numerical aperture objectives. This allows high-sensitivity and high-resolution fluorescence microscopy of vitrified samples. We describe and apply a correlative cryo-fluorescence and cryo-electron microscopy workflow together with a fiducial bead-based image correlation procedure. This procedure allows us to locate fluorescent bacteriophages in cryo-electron microscopy images with an accuracy on the order of 50 nm, based on their fluorescent signal. It will allow the user to precisely and unambiguously identify and locate objects and events for subsequent high-resolution structural study, based on fluorescent signals.
Keywords: Bacteriophage particles; Correlative light and electron microscopy (CLEM); Cryo-electron microscopy (cryo-EM); Cryo-fluorescence microscopy (cryo-FM); Fiducial beads; High-accuracy localization; Low-temperature fluorescence microscopy.
© 2013 Published by Elsevier B.V.
Figures



References
-
- Tsien RY. The green fluorescent protein. Annu Rev Biochem. 1998;67:509–544. - PubMed
-
- Giepmans BNG, Adams SR, Ellisman MH, Tsien RY. The fluorescent toolbox for assessing protein location and function. Science. 2006;312:217–224. - PubMed
-
- Betzig E, Patterson GH, Sougrat R, Lindwasser OW, Olenych S, Bonifacino JS, et al. Imaging intracellular fluorescent proteins at nanometer resolution. Science. 2006;313:1642–1645. - PubMed
-
- Karreman MA, Agronskaia AV, van Donselaar EG, Vocking K, Fereidouni F, Humbel BM, et al. Optimizing immuno-labeling for correlative fluorescence and electron microscopy on a single specimen. J Struct Biol. 2012;180:382–386. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

