Investigations of the mode of action and resistance development of cadazolid, a new antibiotic for treatment of Clostridium difficile infections
- PMID: 24277035
- PMCID: PMC3910891
- DOI: 10.1128/AAC.01831-13
Investigations of the mode of action and resistance development of cadazolid, a new antibiotic for treatment of Clostridium difficile infections
Abstract
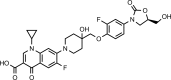
Cadazolid is a new oxazolidinone-type antibiotic currently in clinical development for the treatment of Clostridium difficile-associated diarrhea. Here, we report investigations on the mode of action and the propensity for spontaneous resistance development in C. difficile strains. Macromolecular labeling experiments indicated that cadazolid acts as a potent inhibitor of protein synthesis, while inhibition of DNA synthesis was also observed, albeit only at substantially higher concentrations of the drug. Strong inhibition of protein synthesis was also obtained in strains resistant to linezolid, in agreement with low MICs against such strains. Inhibition of protein synthesis was confirmed in coupled transcription/translation assays using extracts from different C. difficile strains, including strains resistant to linezolid, while inhibitory effects in DNA topoisomerase assays were weak or not detectable under the assay conditions. Spontaneous resistance frequencies of cadazolid were low in all strains tested (generally <10(-10) at 2× to 4× the MIC), and in multiple-passage experiments (up to 13 passages) MICs did not significantly increase. Furthermore, no cross-resistance was observed, as cadazolid retained potent activity against strains resistant or nonsusceptible to linezolid, fluoroquinolones, and the new antibiotic fidaxomicin. In conclusion, the data presented here indicate that cadazolid acts primarily by inhibition of protein synthesis, with weak inhibition of DNA synthesis as a potential second mode of action, and suggest a low potential for spontaneous resistance development.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

