Heme triggers TLR4 signaling leading to endothelial cell activation and vaso-occlusion in murine sickle cell disease
- PMID: 24277079
- PMCID: PMC3894494
- DOI: 10.1182/blood-2013-04-495887
Heme triggers TLR4 signaling leading to endothelial cell activation and vaso-occlusion in murine sickle cell disease
Abstract
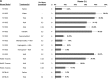
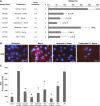
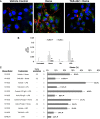
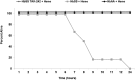
Treatment of sickle cell disease (SCD) is hampered by incomplete understanding of pathways linking hemolysis to vaso-occlusion. We investigated these pathways in transgenic sickle mice. Infusion of hemoglobin or heme triggered vaso-occlusion in sickle, but not normal, mice. Methemoglobin, but not heme-stabilized cyanomethemoglobin, induced vaso-occlusion, indicating heme liberation is necessary. In corroboration, hemoglobin-induced vaso-occlusion was blocked by the methemoglobin reducing agent methylene blue, haptoglobin, or the heme-binding protein hemopexin. Untreated HbSS mice, but not HbAA mice, exhibited ∼10% vaso-occlusion in steady state that was inhibited by haptoglobin or hemopexin infusion. Antibody blockade of adhesion molecules P-selectin, von Willebrand factor (VWF), E-selectin, vascular cell adhesion molecule 1, intercellular adhesion molecule 1, platelet endothelial cell (EC) adhesion molecule 1, α4β1, or αVβ3 integrin prevented vaso-occlusion. Heme rapidly (5 minutes) mobilized Weibel-Palade body (WPB) P-selectin and VWF onto EC and vessel wall surfaces and activated EC nuclear factor κB (NF-κB). This was mediated by TLR4 as TAK-242 blocked WPB degranulation, NF-κB activation, vaso-occlusion, leukocyte rolling/adhesion, and heme lethality. TLR4(-/-) mice transplanted with TLR4(+/+) sickle bone marrow exhibited no heme-induced vaso-occlusion. The TLR4 agonist lipopolysaccharide (LPS) activated ECs and triggered vaso-occlusion that was inhibited by TAK-242, linking hemolysis- and infection-induced vaso-occlusive crises to TLR4 signaling. Heme and LPS failed to activate VWF and NF-κB in TLR4(-/-) ECs. Anti-LPS immunoglobulin G blocked LPS-induced, but not heme-induced, vaso-occlusion, illustrating LPS-independent TLR4 signaling by heme. Inhibition of protein kinase C, NADPH oxidase, or antioxidant treatment blocked heme-mediated stasis, WPB degranulation, and oxidant production. We conclude that intravascular hemolysis in SCD releases heme that activates endothelial TLR4 signaling leading to WPB degranulation, NF-κB activation, and vaso-occlusion.
Figures






References
-
- Graça-Souza AV, Arruda MA, de Freitas MS, Barja-Fidalgo C, Oliveira PL. Neutrophil activation by heme: implications for inflammatory processes. Blood. 2002;99(11):4160–4165. - PubMed
-
- Porto BN, Alves LS, Fernández PL, et al. Heme induces neutrophil migration and reactive oxygen species generation through signaling pathways characteristic of chemotactic receptors. J Biol Chem. 2007;282(33):24430–24436. - PubMed
-
- Wagener FA, Volk HD, Willis D, et al. Different faces of the heme-heme oxygenase system in inflammation. Pharmacol Rev. 2003;55(3):551–571. - PubMed
-
- Figueiredo RT, Fernandez PL, Mourao-Sa DS, et al. Characterization of heme as activator of Toll-like receptor 4. J Biol Chem. 2007;282(28):20221–20229. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

