Broadly neutralizing antibodies that inhibit HIV-1 cell to cell transmission
- PMID: 24277152
- PMCID: PMC3865481
- DOI: 10.1084/jem.20131244
Broadly neutralizing antibodies that inhibit HIV-1 cell to cell transmission
Abstract
The neutralizing activity of anti-HIV-1 antibodies is typically measured in assays where cell-free virions enter reporter cell lines. However, HIV-1 cell to cell transmission is a major mechanism of viral spread, and the effect of the recently described broadly neutralizing antibodies (bNAbs) on this mode of transmission remains unknown. Here we identify a subset of bNAbs that inhibit both cell-free and cell-mediated infection in primary CD4(+) lymphocytes. These antibodies target either the CD4-binding site (NIH45-46 and 3BNC60) or the glycan/V3 loop (10-1074 and PGT121) on HIV-1 gp120 and act at low concentrations by inhibiting multiple steps of viral cell to cell transmission. These antibodies accumulate at virological synapses and impair the clustering and fusion of infected and target cells and the transfer of viral material to uninfected T cells. In addition, they block viral cell to cell transmission to plasmacytoid DCs and thereby interfere with type-I IFN production. Thus, only a subset of bNAbs can efficiently prevent HIV-1 cell to cell transmission, and this property should be considered an important characteristic defining antibody potency for therapeutic or prophylactic antiviral strategies.
Figures

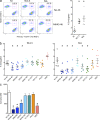
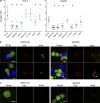
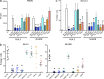
References
-
- Bonsignori M., Montefiori D.C., Wu X., Chen X., Hwang K.K., Tsao C.Y., Kozink D.M., Parks R.J., Tomaras G.D., Crump J.A., et al. 2012. Two distinct broadly neutralizing antibody specificities of different clonal lineages in a single HIV-1-infected donor: implications for vaccine design. J. Virol. 86:4688–4692 10.1128/JVI.07163-11 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

