Cladribine with immediate rituximab for the treatment of patients with variant hairy cell leukemia
- PMID: 24277451
- PMCID: PMC3867590
- DOI: 10.1158/1078-0432.CCR-13-1752
Cladribine with immediate rituximab for the treatment of patients with variant hairy cell leukemia
Abstract
Purpose: In contrast with the classic form, variant hairy cell leukemia (HCLv) responds poorly to single-agent purine analogs, expresses unmutated BRAF, has shorter overall survival, and lacks effective standard therapy. No treatment has achieved a high complete remission (CR) rate even in small series, and of 39 reported cases from six studies, overall response rate after cladribine was 44% with 8% CRs. Rituximab has been found to increase the sensitivity of malignant cells to cladribine, suggesting that combination with cladribine might improve response in HCLv. To test this hypothesis, patients with HCLv were treated with simultaneous cladribine and rituximab.
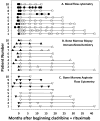
Experimental design: Patients with HCLv with 0 to 1 prior courses of cladribine received cladribine 0.15 mg/kg for days 1 to 5, with eight weekly doses of rituximab 375 mg/m(2) beginning day 1. Restaging was performed, and minimal residual disease (MRD) in blood and marrow was quantified using PCR, immunohistochemistry, and flow cytometry.
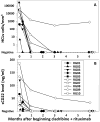
Results: By 6 months, 9 (90%) of 10 patients achieved CR, compared with 3 (8%) of 39 reported cases treated with cladribine alone (P < 0.0001). Of the 9 CRs, 8 remain free of MRD at 12 to 48 (median 27) months of follow-up. No dose-limiting toxicities were observed when beginning cladribine and rituximab on the same day, although most patients required short-term steroids to prevent and treat rituximab infusion reactions. Cytopenias in CRs resolved in 7 to 211 (median 34) days without major infections.
Conclusion: Although cladribine alone lacks effectiveness for early or relapsed HCLv, cladribine with immediate rituximab achieves CRs without MRD and is feasible to administer.
©2013 AACR.
Figures

References
-
- Cawley JC, Burns GF, Hayhoe FG. A chronic lymphoproliferative disorder with distinctive features: a distinct variant of hairy-cell leukaemia. Leuk Res. 1980;4:547–59. - PubMed
-
- Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4. Vol. 2. World Health Organization; 2008.
-
- Grever M, Kopecky K, Foucar MK, Head D, Bennett JM, Hutchison RE, et al. Randomized comparison of pentostatin versus interferon alfa-2a in previously untreated patients with hairy cell leukemia: an intergroup study. J Clin Oncol. 1995;13:974–82. - PubMed
-
- Saven A, Burian C, Koziol JA, Piro LD. Long-term follow-up of patients with hairy cell leukemia after cladribine treatment. Blood. 1998;92:1918–26. - PubMed
-
- Else M, Dearden CE, Matutes E, Garcia-Talavera J, Rohatiner AZ, Johnson SA, et al. Long-term follow-up of 233 patients with hairy cell leukaemia, treated initially with pentostatin or cladribine, at a median of 16 years from diagnosis. Br J Haematol. 2009;145:733–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

