Integrin-mediated cell surface recruitment of autotaxin promotes persistent directional cell migration
- PMID: 24277575
- PMCID: PMC3898650
- DOI: 10.1096/fj.13-232868
Integrin-mediated cell surface recruitment of autotaxin promotes persistent directional cell migration
Abstract
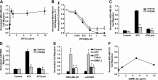
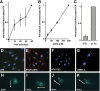
Autotaxin (ATX) is a secreted lysophospholipase D (lysoPLD) that binds to integrin adhesion receptors. We dissected the roles of integrin binding and lysoPLD activity in stimulation of human breast cancer and mouse aortic vascular smooth muscle cell migration by ATX. We compared effects of wild-type human ATX, catalytically inactive ATX, an integrin binding-defective ATX variant with wild-type lysoPLD activity, the isolated ATX integrin binding N-terminal domain, and a potent ATX selective lysoPLD inhibitor on cell migration using transwell and single-cell tracking assays. Stimulation of transwell migration was reduced (18 or 27% of control, respectively) but not ablated by inactivation of integrin binding or inhibition of lysoPLD activity. The N-terminal domain increased transwell migration (30% of control). ATX lysoPLD activity and integrin binding were necessary for a 3.8-fold increase in the fraction of migrating breast cancer cell step velocities >0.7 μm/min. ATX increased the persistent directionality of single-cell migration 2-fold. This effect was lysoPLD activity independent and recapitulated by the integrin binding N-terminal domain. Integrin binding enables uptake and intracellular sequestration of ATX, which redistributes to the front of migrating cells. ATX binding to integrins and lysoPLD activity therefore cooperate to promote rapid persistent directional cell migration.
Keywords: cell adhesion; cell motility; lysophosphatidic acid; lysophospholipase.
Figures



Similar articles
-
Autotaxin induces lung epithelial cell migration through lysoPLD activity-dependent and -independent pathways.Biochem J. 2011 Oct 1;439(1):45-55. doi: 10.1042/BJ20110274. Biochem J. 2011. PMID: 21696367 Free PMC article.
-
Binding of autotaxin to integrins localizes lysophosphatidic acid production to platelets and mammalian cells.J Biol Chem. 2011 Oct 7;286(40):34654-63. doi: 10.1074/jbc.M111.276725. Epub 2011 Aug 10. J Biol Chem. 2011. PMID: 21832043 Free PMC article.
-
Submucosal connective tissue-type mast cells contribute to the production of lysophosphatidic acid (LPA) in the gastrointestinal tract through the secretion of autotaxin (ATX)/lysophospholipase D (lysoPLD).Virchows Arch. 2007 Jul;451(1):47-56. doi: 10.1007/s00428-007-0425-4. Epub 2007 Jun 7. Virchows Arch. 2007. PMID: 17554559
-
Autotaxin: structure-function and signaling.J Lipid Res. 2014 Jun;55(6):1010-8. doi: 10.1194/jlr.R046391. Epub 2014 Feb 18. J Lipid Res. 2014. PMID: 24548887 Free PMC article. Review.
-
Lysophospholipase D and its role in LPA production.Cell Signal. 2004 Sep;16(9):975-81. doi: 10.1016/j.cellsig.2004.03.005. Cell Signal. 2004. PMID: 15212758 Review.
Cited by
-
Hypoxia Downregulates LPP3 and Promotes the Spatial Segregation of ATX and LPP1 During Cancer Cell Invasion.Cancers (Basel). 2019 Sep 19;11(9):1403. doi: 10.3390/cancers11091403. Cancers (Basel). 2019. PMID: 31546971 Free PMC article.
-
The involvement of autotaxin in renal interstitial fibrosis through regulation of fibroblast functions and induction of vascular leakage.Sci Rep. 2019 May 15;9(1):7414. doi: 10.1038/s41598-019-43576-x. Sci Rep. 2019. PMID: 31092842 Free PMC article.
-
Platelets, autotaxin and lysophosphatidic acid signalling: win-win factors for cancer metastasis.Br J Pharmacol. 2018 Aug;175(15):3100-3110. doi: 10.1111/bph.14362. Epub 2018 Jun 25. Br J Pharmacol. 2018. PMID: 29777586 Free PMC article. Review.
-
The Expression Regulation and Biological Function of Autotaxin.Cells. 2021 Apr 19;10(4):939. doi: 10.3390/cells10040939. Cells. 2021. PMID: 33921676 Free PMC article. Review.
-
Lysophosphatidic acid produced by autotaxin acts as an allosteric modulator of its catalytic efficiency.J Biol Chem. 2018 Sep 14;293(37):14312-14327. doi: 10.1074/jbc.RA118.004450. Epub 2018 Jul 19. J Biol Chem. 2018. PMID: 30026231 Free PMC article.
References
-
- Stracke M. L., Krutzsch H. C., Unsworth E. J., Arestad A., Cioce V., Schiffmann E., Liotta L. A. (1992) Identification, purification, and partial sequence analysis of autotaxin, a novel motility-stimulating protein. J. Biol. Chem. 267, 2524–2529 - PubMed
-
- Houben A. J., Moolenaar W. H. (2011) Autotaxin and LPA receptor signaling in cancer. Cancer Metastasis Rev. 30, 557–565 - PubMed
-
- Moolenaar W. H., Perrakis A. (2011) Insights into autotaxin: how to produce and present a lipid mediator. Nat. Rev. Mol. Cell Biol. 12, 674–679 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

