Prophylactic interventions after delivery of placenta for reducing bleeding during the postnatal period
- PMID: 24277681
- PMCID: PMC11973543
- DOI: 10.1002/14651858.CD009328.pub2
Prophylactic interventions after delivery of placenta for reducing bleeding during the postnatal period
Abstract
Background: There are several Cochrane systematic reviews looking at postpartum haemorrhage (PPH) prophylaxis in the third stage of labour and another Cochrane review investigating the timing of prophylactic uterotonics in the third stage of labour (i.e. before or after delivery of the placenta). There are, however, no Cochrane reviews looking at the use of interventions given purely after delivery of the placenta. Ergometrine or methylergometrine are used for the prevention of PPH in the postpartum period (the period after delivery of the infant) after delivery of the placenta in some countries. There are, furthermore, no Cochrane reviews that have so far considered herbal therapies or homeopathic remedies for the prevention of PPH after delivery of the placenta.
Objectives: To assess the effectiveness of available prophylactic interventions for PPH including prophylactic use of ergotamine, ergometrine, methylergometrine, herbal therapies, and homeopathic remedies, administered after delivery of the placenta, compared with no uterotonic agents as well as with different routes of administration for prevention of PPH after delivery of the placenta.
Search methods: We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (30 April 2013), The Food and Drug Administration (FDA) (USA), Medicines and Healthcare Products Regulatory Agency (MHRA) (UK), European Medicines Agency (EMA) (EU), Pharmaceuticals and Medical Devices Agency (PMDA) (Japan), Therapeutic Goods Administration (TGA) (Australia), ClinicalTrials.gov, Current Controlled Trials, WHO International Clinical Trials Registry Platform (ICTRP), University Hospital Medical Information Network Clinical Trials Registry (UMIN-CTR; Japan), Japan Pharmaceutical Information Center Clinical Trials Information (Japic-CTI; Japan), Japan Medical Association Clinical Trial Registration (JMACCT CTR; Japan) (all on 30 April 2013) and reference lists of retrieved studies
Selection criteria: All randomised or quasi-randomised controlled trials comparing prophylactic ergotamine, ergometrine, methylergometrine, herbal therapies, and homeopathic remedies (using any route and timing of administration) during the postpartum period after delivery of the placenta with no uterotonic agents or trials comparing different routes or timing of administration of ergotamine, ergometrine, methylergometrine, herbal therapies, and homeopathic remedies, during the postpartum period after delivery of the placenta.
Data collection and analysis: Two review authors independently assessed trial eligibility and the methodological quality of trials, extracted data using the agreed form. Data were checked for accuracy.
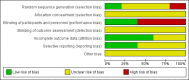
Main results: Five randomised studies involving 1466 women met the inclusion criteria. All studies were classified as having an unclear risk of bias. Two studies (involving 1097 women) compared oral methylergometrine with a placebo, and one (involving 171 women) compared oral methylergometrine with Kyuki-chouketsu-in, a Japanese traditional herbal medicine. The remaining two studies (involving 198 women) did not report the outcomes of interest for this review. None of the included studies reported primary outcomes prespecified in the review protocol (blood loss of 1000 mL or more over the period of observation, maternal death or severe morbidity). Overall, there was no clear evidence of differences between groups in the following PPH outcomes: blood loss of 500 mL or more (risk ratio (RR) 1.45; 95% confidence interval (CI) 0.39 to 5.47, two studies), amount of lochia during the first 72 hours of the puerperium (mean difference (MD) -25.00 g; 95% CI -69.79 to 19.79, one study), or amount of lochia by four weeks postpartum (MD -7.00 g; 95% CI -23.99 to 9.99).The Japanese study with a relatively small sample size comparing oral methylergometrine with a Japanese traditional herbal medicine found that oral methylergometrine significantly increased the blood haemoglobin concentration at day one postpartum (MD 0.50 g/dL; 95% CI 0.11 to 0.89) compared to herbal medicine. Adverse events were not well-reported in the included studies. We did not find any studies comparing homeopathic remedies with either a placebo or no treatment.
Authors' conclusions: There was insufficient evidence to support the use of prophylactic oral methylergometrine given after delivery of the placenta for the prevention of PPH. Additionally, the effectiveness of prophylactic use of herbal medicine or homeopathic remedies for PPH is still unclear as we could not find any clear evidence. Trials to assess the effectiveness of herbal medicines and homeopathic remedies in preventing PPH are warranted.
Conflict of interest statement
None known.
Figures








Update of
- doi: 10.1002/14651858.CD009328
References
References to studies included in this review
Andersen 1998 {published data only}
-
- Andersen B, Andersen LLT, Sorensen T. Methylergometrine during the early puerperium; a prospective randomized double blind study. Acta Obstetricia et Gynecologica Scandinavica 1998;77:54‐7. - PubMed
Arabin 1986 {published data only}
-
- Arabin B, Ruttgers H, Kubli F. Effects of routine administration of methylergometrine during the puerperium on involution, maternal morbidity, and lactation. Geburtshilfe und Frauenheilkunde 1986;46:215‐20. - PubMed
Jolivet 1978 {published data only}
-
- Jolivet A, Robyn C, Huraux‐Rendu C, Gautray JP. Effect of ergot alkaloid derivatives on milk secretion in the immediate postpartum period. Journal de Gynecologie, Obstetrique et Biologie de la Reproduction 1978;7:129‐34. - PubMed
Klug 1983 {published data only}
-
- Klug PW, Mayer HGK. Influence of methyl‐ergotamine on early puerperal involution of the uterus. Zeitschrift fur Geburtshilfe und Perinatologie 1983;187:203‐4. - PubMed
Ushiroyama 2003 {published data only}
-
- Ushiroyama T, Sakuma K, Souen H, Nakai G, Morishima S, Yasuda K, et al. Therapeutic effects of Kyuki‐chouketsu‐in in restoring postpartum physical condition. American Journal of Chinese Medicine 2003;31(3):437‐44. - PubMed
References to studies excluded from this review
Del Pozo 1975 {published data only}
-
- Pozo E, Brun Del Re R, Hinselman M. Lack of effect of methyl‐ergonovine on postpartum lactation. American Journal of Obstetrics and Gynecology 1975;123:845‐6. - PubMed
Vogel 2004 {published data only}
-
- Vogel D, Burkhardt T, Rentsch K, Schweer H, Watzer B, Zimmerman R, et al. Misoprostol versus methylergometrine: pharmacokinetics in human milk. American Journal of Obstetrics and Gynecology 2004;191:2168‐73. - PubMed
References to studies awaiting assessment
Arabin 1982 {published data only}
-
- Arabin B, Ruttgers H, Kubli F. Influence of a routine use of methergine on puerperal infection and breast feeding. Proceedings of 8th European Congress of Perinatal Medicine; 1982 Sept 7‐10; Brussels, Belgium. 1982:Abstract no: 81.
Grünberger 1983 {published data only}
-
- Grünberger W. Postpartum uterus involution and lactation levels in randomized comparison between prostin E2 tablets and methergine dragees [Postpartale Uterusinvolution und Laktationsmengen im randomisierten Vergleich von Prostin‐E2‐Tabletten mit Methergin‐Dragees]. Gynakologische Rundschau 1983;23(2):100‐7. - PubMed
References to ongoing studies
Oberbaum 2005 {published data only}
-
- Oberbaum M, Galoyan N, Lerner‐Geva L, Singer SR, Grisaru S, Shashar D, et al. The effect of the homeopathic remedies Arnica montana and Bellis perennis on mild postpartum bleeding‐a randomized, double‐blind, placebo‐controlled study‐ preliminary results. Complementary Therapies in Medicine 2005;13(2):87‐90. - PubMed
-
- Samuels N, Oberbaum M. The effect of the homoeopathic remedies Arnica montana and Bellis perennis on postpartum bleeding ‐ a randomised, double‐blind, placebo‐controlled study [abstract]. Focus on Alternative and Complementary Therapies 2005;10 Suppl 1:47. - PubMed
Additional references
ACOG 2006
-
- American College of Obstetricians and Gynecologists. Postpartum Hemorrhage. Practice Bulletin Number 76. ACOG, October 2006.
Aeby 2003
-
- Aeby A, Johansson AB, Schuiteneer B, Blum D. Methylergometrine poisoning in children: review of 34 cases. Journal of Toxicology Clinical Toxicology 2003;41(3):249‐53. - PubMed
Begley 2011
Brucker 2001
-
- Brucker MC. Management of the third stage of labor: an evidence‐based approach. Journal of Midwifery & Women’s Health 2001;46(6):381‐92. - PubMed
Cameron 2006
-
- Cameron CA, Roberts CL, Olive EC, Ford JB, Fischer WE. Trends in postpartum haemorrhage. Australian and New Zealand Journal of Public Health 2006;30(2):151‐6. - PubMed
Cotter 2010
Cunningham 2010
-
- Cunningham FG, Leveno KJ, Boom SL, Hauth JC, Rouse DJ, Spong CY, editors. The Puerperium. In: Williams Obstetrics. 23rd Edition. New York, NY: McGraw‐Hill, 2010. p. 646:646.
De Groot 1996a
-
- Groot AN, Roosmalen J, Dongen PW, Borm GF. A placebo‐controlled trial of oral ergometrine to reduce postpartum hemorrhage. Acta Obstetricia et Gynecologica Scandinavica 1996;75(5):464‐8. - PubMed
De Groot 1996b
-
- Groot AN. The role of oral (methyl)ergometrine in the prevention of postpartum haemorrhage. European Journal of Obstetrics & Gynecology and Reproductive Biology 1996;69(1):31‐6. - PubMed
De Groot 1998
-
- Groot AN, Dongen PW, Vree TB, Hekster YA, Roosmalen J. Ergot alkaloids. Current status and review of clinical pharmacology and therapeutic use compared with other oxytocics in obstetrics and gynaecology. Drugs 1998;56(4):523‐35. - PubMed
Ford 2007
-
- Ford JB, Roberts CL, Simpson JM, Vaughan J, Cameron CA. Increased postpartum hemorrhage rates in Australia. International Journal of Gynecology & Obstetrics 2007;98(3):237‐43. - PubMed
Gates 2005
-
- Gates S. Methodological Guidelines. In: The Editorial Team. Pregnancy and Childbirth Group. About the Cochrane Collaboration (Collaborative Review Groups (CRGs)) 2005, Issue 2.
Higgins 2011
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hofmeyr 2010
-
- Hofmeyr GJ, Abdel‐Aleem H, Abdel‐Aleem MA. Uterine massage for preventing postpartum haemorrhage. Cochrane Database of Systematic Reviews 2010, Issue 8. [DOI: 10.1002/14651858.CD006431.pub2] - DOI
ICM‐FIGO 2003
-
- International Confederation of Midwives (ICM) and International Federation of Gynaecology and Obstetrics (FIGO). Joint statement. Management of the third stage of labour to prevent post‐partum haemorrhage. http://www.figo.org/files/figo‐corp/docs/PPH%20Joint%20Statement.pdf (accessed 2 June 2011).
ICM‐FIGO 2006
-
- International Confederation of Midwives (ICM) and International Federation of Gynaecology and Obstetrics (FIGO). Prevention and treatment of post‐partum haemorrhage: new advances for low resource settings. Joint statement. http://www.figo.org/files/figo‐corp/docs/PPH%20Joint%20Statement%202%20E... (accessed 2 June 2011).
Joseph 2007
-
- Joseph KS, Rouleau J, Kramer MS, Young DC, Liston RM, Baskett TF, et al. Investigation of an increase in postpartum haemorrhage in Canada. BJOG: an international journal of obstetrics and gynaecology 2007;114(6):751‐9. - PubMed
Knight 2009
Liabsuetrakul 2011
McDonald 2009
Mori 2012
Novikova 2011
Okada 2005
-
- Okada J, Takemura N, Murakoshi H, Ide K, Nakajima K, Kitagaki S. Examination of methylergometrine use during postpartum period in our hospital. Aijinkai Medical Journal 2005;36:20‐1.
Oladapo 2012
Peña‐Martí 2010
RevMan 2011 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Rohwer 2012
Soltani 2010
Soltani 2011
Su 2012
Tunçalp 2012
Van Selm 1995
-
- Selm M, Kanhai HH, Keirse MJ. Preventing the recurrence of atonic postpartum hemorrhage: a double‐blind trial. Acta Obstetricia et Gynecologica Scandinavica 1995;74(4):270‐4. - PubMed
WHO 2005
-
- World Health Organization. The World Health Report 2005: Make Every Mother and Child Count. Geneva: World Health Organization, 2005.
WHO 2009
-
- World Health Organization. WHO Guidelines for the Management of Postpartum Haemorrhage and Retained Placenta. Geneva: World Health Organization, 2009.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

