Self-organization of axial polarity, inside-out layer pattern, and species-specific progenitor dynamics in human ES cell-derived neocortex
- PMID: 24277810
- PMCID: PMC3864329
- DOI: 10.1073/pnas.1315710110
Self-organization of axial polarity, inside-out layer pattern, and species-specific progenitor dynamics in human ES cell-derived neocortex
Erratum in
- Proc Natl Acad Sci U S A. 2014 May 20;111(20):7498
Abstract
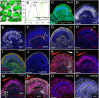
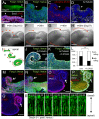
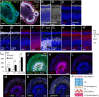
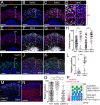
Here, using further optimized 3D culture that allows highly selective induction and long-term growth of human ES cell (hESC)-derived cortical neuroepithelium, we demonstrate unique aspects of self-organization in human neocorticogenesis. Self-organized cortical tissue spontaneously forms a polarity along the dorsocaudal-ventrorostral axis and undergoes region-specific rolling morphogenesis that generates a semispherical structure. The neuroepithelium self-forms a multilayered structure including three neuronal zones (subplate, cortical plate, and Cajal-Retzius cell zones) and three progenitor zones (ventricular, subventricular, and intermediate zones) in the same apical-basal order as seen in the human fetal cortex in the early second trimester. In the cortical plate, late-born neurons tend to localize more basally to early-born neurons, consistent with the inside-out pattern seen in vivo. Furthermore, the outer subventricular zone contains basal progenitors that share characteristics with outer radial glia abundantly found in the human, but not mouse, fetal brain. Thus, human neocorticogenesis involves intrinsic programs that enable the emergence of complex neocortical features.
Keywords: corticogenesis; stratification.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Molyneaux BJ, Arlotta P, Menezes JR, Macklis JD. Neuronal subtype specification in the cerebral cortex. Nat Rev Neurosci. 2007;8(6):427–437. - PubMed
-
- Bielle F, et al. Multiple origins of Cajal-Retzius cells at the borders of the developing pallium. Nat Neurosci. 2005;8(8):1002–1012. - PubMed
-
- Bystron I, Blakemore C, Rakic P. Development of the human cerebral cortex: Boulder Committee revisited. Nat Rev Neurosci. 2008;9(2):110–122. - PubMed
-
- Rakic P. Neurons in rhesus monkey visual cortex: Systematic relation between time of origin and eventual disposition. Science. 1974;183(4123):425–427. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

