Autosis is a Na+,K+-ATPase-regulated form of cell death triggered by autophagy-inducing peptides, starvation, and hypoxia-ischemia
- PMID: 24277826
- PMCID: PMC3870705
- DOI: 10.1073/pnas.1319661110
Autosis is a Na+,K+-ATPase-regulated form of cell death triggered by autophagy-inducing peptides, starvation, and hypoxia-ischemia
Abstract
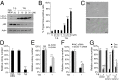
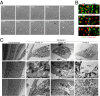
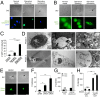
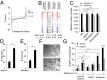
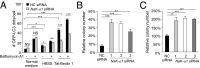
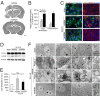
A long-standing controversy is whether autophagy is a bona fide cause of mammalian cell death. We used a cell-penetrating autophagy-inducing peptide, Tat-Beclin 1, derived from the autophagy protein Beclin 1, to investigate whether high levels of autophagy result in cell death by autophagy. Here we show that Tat-Beclin 1 induces dose-dependent death that is blocked by pharmacological or genetic inhibition of autophagy, but not of apoptosis or necroptosis. This death, termed "autosis," has unique morphological features, including increased autophagosomes/autolysosomes and nuclear convolution at early stages, and focal swelling of the perinuclear space at late stages. We also observed autotic death in cells during stress conditions, including in a subpopulation of nutrient-starved cells in vitro and in hippocampal neurons of neonatal rats subjected to cerebral hypoxia-ischemia in vivo. A chemical screen of ~5,000 known bioactive compounds revealed that cardiac glycosides, antagonists of Na(+),K(+)-ATPase, inhibit autotic cell death in vitro and in vivo. Furthermore, genetic knockdown of the Na(+),K(+)-ATPase α1 subunit blocks peptide and starvation-induced autosis in vitro. Thus, we have identified a unique form of autophagy-dependent cell death, a Food and Drug Administration-approved class of compounds that inhibit such death, and a crucial role for Na(+),K(+)-ATPase in its regulation. These findings have implications for understanding how cells die during certain stress conditions and how such cell death might be prevented.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Comment in
-
Autosis occurs in the liver of patients with severe anorexia nervosa.Hepatology. 2015 Aug;62(2):657-8. doi: 10.1002/hep.27597. Epub 2015 Jun 11. Hepatology. 2015. PMID: 25376517 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

