Glutaredoxin regulates vascular development by reversible glutathionylation of sirtuin 1
- PMID: 24277839
- PMCID: PMC3864331
- DOI: 10.1073/pnas.1313753110
Glutaredoxin regulates vascular development by reversible glutathionylation of sirtuin 1
Abstract
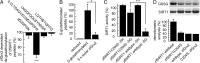
Embryonic development depends on complex and precisely orchestrated signaling pathways including specific reduction/oxidation cascades. Oxidoreductases of the thioredoxin family are key players conveying redox signals through reversible posttranslational modifications of protein thiols. The importance of this protein family during embryogenesis has recently been exemplified for glutaredoxin 2, a vertebrate-specific glutathione-disulfide oxidoreductase with a critical role for embryonic brain development. Here, we discovered an essential function of glutaredoxin 2 during vascular development. Confocal microscopy and time-lapse studies based on two-photon microscopy revealed that morpholino-based knockdown of glutaredoxin 2 in zebrafish, a model organism to study vertebrate embryogenesis, resulted in a delayed and disordered blood vessel network. We were able to show that formation of a functional vascular system requires glutaredoxin 2-dependent reversible S-glutathionylation of the NAD(+)-dependent protein deacetylase sirtuin 1. Using mass spectrometry, we identified a cysteine residue in the conserved catalytic region of sirtuin 1 as target for glutaredoxin 2-specific deglutathionylation. Thereby, glutaredoxin 2-mediated redox regulation controls enzymatic activity of sirtuin 1, a mechanism we found to be conserved between zebrafish and humans. These results link S-glutathionylation to vertebrate development and successful embryonic angiogenesis.
Keywords: cardiovascular system; proteomics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

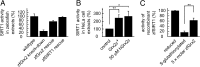


References
-
- Ufer C, Wang CC, Borchert A, Heydeck D, Kuhn H. Redox control in mammalian embryo development. Antioxid Redox Signal. 2010;13(6):833–875. - PubMed
-
- Holmgren A, et al. Thiol redox control via thioredoxin and glutaredoxin systems. Biochem Soc Trans. 2005;33(Pt 6):1375–1377. - PubMed
-
- Lillig CH, Berndt C, Holmgren A. Glutaredoxin systems. Biochimica et Biophysica Acta. 2008;1780(11):1304–1317. - PubMed
-
- Lillig CH, Berndt C. Glutaredoxins in thiol/disulfide exchange. Antioxid Redox Signal. 2013;18(13):1654–1665. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

