SPOC1-mediated antiviral host cell response is antagonized early in human adenovirus type 5 infection
- PMID: 24278021
- PMCID: PMC3836738
- DOI: 10.1371/journal.ppat.1003775
SPOC1-mediated antiviral host cell response is antagonized early in human adenovirus type 5 infection
Abstract
Little is known about immediate phases after viral infection and how an incoming viral genome complex counteracts host cell defenses, before the start of viral gene expression. Adenovirus (Ad) serves as an ideal model, since entry and onset of gene expression are rapid and highly efficient, and mechanisms used 24-48 hours post infection to counteract host antiviral and DNA repair factors (e.g. p53, Mre11, Daxx) are well studied. Here, we identify an even earlier host cell target for Ad, the chromatin-associated factor and epigenetic reader, SPOC1, recently found recruited to double strand breaks, and playing a role in DNA damage response. SPOC1 co-localized with viral replication centers in the host cell nucleus, interacted with Ad DNA, and repressed viral gene expression at the transcriptional level. We discovered that this SPOC1-mediated restriction imposed upon Ad growth is relieved by its functional association with the Ad major core protein pVII that enters with the viral genome, followed by E1B-55K/E4orf6-dependent proteasomal degradation of SPOC1. Mimicking removal of SPOC1 in the cell, knock down of this cellular restriction factor using RNAi techniques resulted in significantly increased Ad replication, including enhanced viral gene expression. However, depletion of SPOC1 also reduced the efficiency of E1B-55K transcriptional repression of cellular promoters, with possible implications for viral transformation. Intriguingly, not exclusive to Ad infection, other human pathogenic viruses (HSV-1, HSV-2, HIV-1, and HCV) also depleted SPOC1 in infected cells. Our findings provide a general model for how pathogenic human viruses antagonize intrinsic SPOC1-mediated antiviral responses in their host cells. A better understanding of viral entry and early restrictive functions in host cells should provide new perspectives for developing antiviral agents and therapies. Conversely, for Ad vectors used in gene therapy, counteracting mechanisms eradicating incoming viral DNA would increase Ad vector efficacy and safety for the patient.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





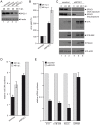


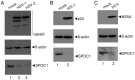
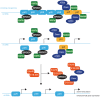
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

