IQ domain GTPase-activating protein 1 is involved in shear stress-induced progenitor-derived endothelial cell alignment
- PMID: 24278215
- PMCID: PMC3838429
- DOI: 10.1371/journal.pone.0079919
IQ domain GTPase-activating protein 1 is involved in shear stress-induced progenitor-derived endothelial cell alignment
Abstract
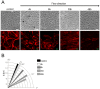
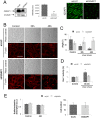
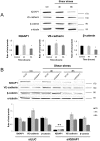
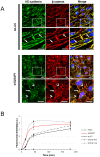
Shear stress is one of mechanical constraints which are exerted by blood flow on endothelial cells (ECs). To adapt to shear stress, ECs align in the direction of flow through adherens junction (AJ) remodeling. However, mechanisms regulating ECs alignment under shear stress are poorly understood. The scaffold protein IQ domain GTPase activating protein 1 (IQGAP1) is a scaffold protein which couples cell signaling to the actin and microtubule cytoskeletons and is involved in cell migration and adhesion. IQGAP1 also plays a role in AJ organization in epithelial cells. In this study, we investigated the potential IQGAP1 involvement in the endothelial cells alignment under shear stress. Progenitor-derived endothelial cells (PDECs), transfected (or not) with IQGAP1 small interfering RNA, were exposed to a laminar shear stress (1.2 N/m(2)) and AJ proteins (VE-cadherin and β-catenin) and IQGAP1 were labeled by immunofluorescence. We show that IQGAP1 is essential for ECs alignment under shear stress. We studied the role of IQGAP1 in AJs remodeling of PDECs exposed to shear stress by studying cell localization and IQGAP1 interactions with VE-cadherin and β-catenin by immunofluorescence and Proximity Ligation Assays. In static conditions, IQGAP1 interacts with VE-cadherin but not with β-catenin at the cell membrane. Under shear stress, IQGAP1 lost its interaction from VE-cadherin to β-catenin. This "switch" was concomitant with the loss of β-catenin/VE-cadherin interaction at the cell membrane. This work shows that IQGAP1 is essential to ECs alignment under shear stress and that AJ remodeling represents one of the mechanisms involved. These results provide a new approach to understand ECs alignment under to shear stress.
Conflict of interest statement
Figures


References
-
- Noria S, Cowan DB, Gotlieb AI, Langille BL (1999) Transient and steady-state effects of shear stress on endothelial cell adherens junctions. Circ Res 85: 504–514. - PubMed
-
- Yamamoto K, Takahashi T, Asahara T, Ohura N, Sokabe T, et al. (2003) Proliferation, differentiation, and tube formation by endothelial progenitor cells in response to shear stress. J Appl Physiol 95: 2081–2088. - PubMed
-
- Ukropec JA, Hollinger MK, Woolkalis MJ (2002) Regulation of VE-cadherin linkage to the cytoskeleton in endothelial cells exposed to fluid shear stress. Exp Cell Res 273: 240–247. - PubMed
-
- Resnick N, Yahav H, Shay-Salit A, Shushy M, Schubert S, et al. (2003) Fluid shear stress and the vascular endothelium: for better and for worse. Prog Biophys Mol Biol 81: 177–199. - PubMed
-
- Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, et al. (2005) A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature 437: 426–431. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

