Heparin modulates the endopeptidase activity of Leishmania mexicana cysteine protease cathepsin L-Like rCPB2.8
- PMID: 24278253
- PMCID: PMC3836952
- DOI: 10.1371/journal.pone.0080153
Heparin modulates the endopeptidase activity of Leishmania mexicana cysteine protease cathepsin L-Like rCPB2.8
Abstract
Background: Cysteine protease B is considered crucial for the survival and infectivity of the Leishmania in its human host. Several microorganism pathogens bind to the heparin-like glycosaminoglycans chains of proteoglycans at host-cell surface to promote their attachment and internalization. Here, we have investigated the influence of heparin upon Leishmania mexicana cysteine protease rCPB2.8 activity.
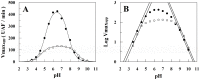
Methodology/principal findings: THE DATA ANALYSIS REVEALED THAT THE PRESENCE OF HEPARIN AFFECTS ALL STEPS OF THE ENZYME REACTION: (i) it decreases 3.5-fold the k 1 and 4.0-fold the k -1, (ii) it affects the acyl-enzyme accumulation with pronounced decrease in k 2 (2.7-fold), and also decrease in k 3 (3.5-fold). The large values of ΔG = 12 kJ/mol for the association and dissociation steps indicate substantial structural strains linked to the formation/dissociation of the ES complex in the presence of heparin, which underscore a conformational change that prevents the diffusion of substrate in the rCPB2.8 active site. Binding to heparin also significantly decreases the α-helix content of the rCPB2.8 and perturbs the intrinsic fluorescence emission of the enzyme. The data strongly suggest that heparin is altering the ionization of catalytic (Cys(25))-S(-)/(His(163))-Im(+) H ion pair of the rCPB2.8. Moreover, the interaction of heparin with the N-terminal pro-region of rCPB2.8 significantly decreased its inhibitory activity against the mature enzyme.
Conclusions/significance: Taken together, depending on their concentration, heparin-like glycosaminoglycans can either stimulate or antagonize the activity of cysteine protease B enzymes during parasite infection, suggesting that this glycoconjugate can anchor parasite cysteine protease at host cell surface.
Conflict of interest statement
Figures





References
-
- Sajid M, McKerrow JH (2002) Cysteine proteases of parasitic organisms. Mol Biochem Parasitol 120: 1-21. Review. Erratum in: Mol Biochem Parasitol 121: 159. - PubMed
-
- Coombs GH (1982) Proteinases of Leishmania mexicana and other flagellate protozoa. Parasitology 84: 149–155. - PubMed
-
- Pupkis MF, Coombs GH (1984) Purification and characterization of proteolytic enzymes of Leishmania mexicana amastigotes and promastigotes. J Gen Microbiol 130: 2375–2383. - PubMed
-
- Robertson CD, Coombs GH (1990) Characterization of three groups of cysteine proteinases in the amastigotes of Leishmania mexicana mexicana . Mol Biochem Parasitol 42: 269–276. - PubMed
-
- Robertson CD, Coombs GH (1992) Stage-specific proteinases of Leishmania mexicana promastigotes. FEMS Microbiol Lett 94: 127–132. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

