Commensal bacteria-dependent indole production enhances epithelial barrier function in the colon
- PMID: 24278294
- PMCID: PMC3835565
- DOI: 10.1371/journal.pone.0080604
Commensal bacteria-dependent indole production enhances epithelial barrier function in the colon
Abstract
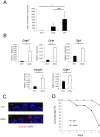
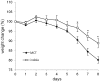
Microbiota have been shown to have a great influence on functions of intestinal epithelial cells (ECs). The role of indole as a quorum-sensing (QS) molecule mediating intercellular signals in bacteria has been well appreciated. However, it remains unknown whether indole has beneficial effects on maintaining intestinal barriers in vivo. In this study, we analyzed the effect of indole on ECs using a germ free (GF) mouse model. GF mice showed decreased expression of junctional complex molecules in colonic ECs. The feces of specific pathogen-free (SPF) mice contained a high amount of indole; however the amount was significantly decreased in the feces of GF mice by 27-fold. Oral administration of indole-containing capsules resulted in increased expression of both tight junction (TJ)- and adherens junction (AJ)-associated molecules in colonic ECs in GF mice. In accordance with the increased expression of these junctional complex molecules, GF mice given indole-containing capsules showed higher resistance to dextran sodium sulfate (DSS)-induced colitis. A similar protective effect of indole on DSS-induced epithelial damage was also observed in mice bred in SPF conditions. These findings highlight the beneficial role of indole in establishing an epithelial barrier in vivo.
Conflict of interest statement
Figures



References
-
- Blachier F, Mariotti F, Huneau JF, Tome D (2007) Effects of amino acid-derived luminal metabolites on the colonic epithelium and physiopathological consequences. Amino Acids 33: 547–562. - PubMed
-
- Tremaroli V, Backhed F (2012) Functional interactions between the gut microbiota and host metabolism. Nature 489: 242–249. - PubMed
-
- Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, et al. (2012) Host-gut microbiota metabolic interactions. Science 336: 1262–1267. - PubMed
-
- Hooper LV, Macpherson AJ (2010) Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat Rev Immunol 10: 159–169. - PubMed
-
- Turner JR (2009) Intestinal mucosal barrier function in health and disease. Nat Rev Immunol 9: 799–809. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

