Nanomechanics of the endothelial glycocalyx in experimental sepsis
- PMID: 24278345
- PMCID: PMC3835794
- DOI: 10.1371/journal.pone.0080905
Nanomechanics of the endothelial glycocalyx in experimental sepsis
Abstract
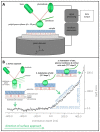
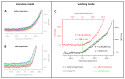
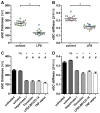
The endothelial glycocalyx (eGC), a carbohydrate-rich layer lining the luminal side of the endothelium, regulates vascular adhesiveness and permeability. Although central to the pathophysiology of vascular barrier dysfunction in sepsis, glycocalyx damage has been generally understudied, in part because of the aberrancy of in vitro preparations and its degradation during tissue handling. The aim of this study was to analyze inflammation-induced damage of the eGC on living endothelial cells by atomic-force microscopy (AFM) nanoindentation technique. AFM revealed the existence of a mature eGC on the luminal endothelial surface of freshly isolated rodent aorta preparations ex vivo, as well as on cultured human pulmonary microvascular endothelial cells (HPMEC) in vitro. AFM detected a marked reduction in glycocalyx thickness (266 ± 12 vs. 137 ± 17 nm, P<0.0001) and stiffness (0.34 ± 0.03 vs. 0.21 ± 0.01 pN/mn, P<0.0001) in septic mice (1 mg E. coli lipopolysaccharides (LPS)/kg BW i.p.) compared to controls. Corresponding in vitro experiments revealed that sepsis-associated mediators, such as thrombin, LPS or Tumor Necrosis Factor-α alone were sufficient to rapidly decrease eGC thickness (-50%, all P<0.0001) and stiffness (-20% P<0.0001) on HPMEC. In summary, AFM nanoindentation is a promising novel approach to uncover mechanisms involved in deterioration and refurbishment of the eGC in sepsis.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

