The Nogo-B-PirB axis controls macrophage-mediated vascular remodeling
- PMID: 24278366
- PMCID: PMC3835671
- DOI: 10.1371/journal.pone.0081019
The Nogo-B-PirB axis controls macrophage-mediated vascular remodeling
Abstract
Objective: Nogo-B mediates vascular protection and facilitates monocyte- and macrophage-dependent vascular remodeling. PirB is an alternate receptor for Nogo-B, but a role for the Nogo-PirB axis within the vascular system has not been previously reported. We examined whether Nogo-B or PirB play a role in regulating macrophage-mediated vascular remodeling and hypothesized that endothelial Nogo-B regulates vein graft macrophage infiltration via its alternate receptor PirB.
Methods: Vein grafts were performed using Nogo and PirB wild type and knockout mice. Human vein grafts were similarly analyzed. The hindlimb ischemia model was performed in PirB wild type and knockout mice. Accompanying in vitro work included isolation of macrophages from PirB wild type and knockout mice.
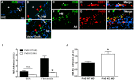
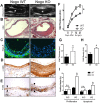
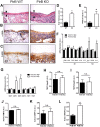
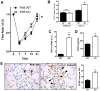
Results: Increased Nogo-B and PirB mRNA transcripts and protein expression were observed within mouse and human vein grafts. Both Nogo knockout and PirB knockout vein grafts showed increased wall thickness and increased numbers of F4/80-positive macrophages. Macrophages derived from PirB knockout mice had increased adhesion to fibronectin, increased EC-specific binding, and increased numbers of mRNA transcripts of M2 markers as well as MMP3 and MMP9. PirB knockout vein grafts had increased active MMP9 compared to wild type vein grafts. PirB knockout mice had increased recovery from hindlimb ischemia and increased macrophage infiltration compared to wild type mice.
Conclusions: Vein graft adaptation shows increased expression of both Nogo-B and PirB. Loss of PirB, or its endothelial ligand Nogo-B, results in increased inflammatory cell infiltration and vein graft wall thickening. These findings suggest that PirB regulates macrophage activity in vein grafts and that Nogo-B in the vein graft limits macrophage infiltration and vein graft thickening. PirB may play a more general role in regulating macrophage responses to vascular injury. Macrophage inhibition via Nogo-PirB interactions may be an important mechanism regulating vein graft adaptation to the arterial circulation.
Conflict of interest statement
Figures

References
-
- Acevedo L, Yu J, Erdjument-Bromage H, Miao RQ, Kim JE, Fulton D (2004) A new role for Nogo as a regulator of vascular remodeling. Nat Med 10: 382–388. - PubMed
-
- Kudo FA, Muto A, Maloney SP, Pimiento JM, Bergaya S, et al. (2007) Venous identity is lost but arterial identity is not gained during vein graft adaptation. Arterioscler Thromb Vasc Biol 27: 1562–1571. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

