A novel in vitro model for microvasculature reveals regulation of circumferential ECM organization by curvature
- PMID: 24278378
- PMCID: PMC3836741
- DOI: 10.1371/journal.pone.0081061
A novel in vitro model for microvasculature reveals regulation of circumferential ECM organization by curvature
Abstract
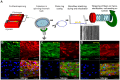
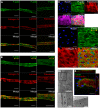
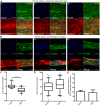
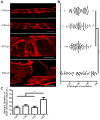
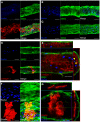
In microvascular vessels, endothelial cells are aligned longitudinally whereas several components of the extracellular matrix (ECM) are organized circumferentially. While current three-dimensional (3D) in vitro models for microvasculature have allowed the study of ECM-regulated tubulogenesis, they have limited control over topographical cues presented by the ECM and impart a barrier for the high-resolution and dynamic study of multicellular and extracellular organization. Here we exploit a 3D fibrin microfiber scaffold to develop a novel in vitro model of the microvasculature that recapitulates endothelial alignment and ECM deposition in a setting that also allows the sequential co-culture of mural cells. We show that the microfibers' nanotopography induces longitudinal adhesion and alignment of endothelial colony-forming cells (ECFCs), and that these deposit circumferentially organized ECM. We found that ECM wrapping on the microfibers is independent of ECFCs' actin and microtubule organization, but it is dependent on the curvature of the microfiber. Microfibers with smaller diameters (100-400 µm) guided circumferential ECM deposition, whereas microfibers with larger diameters (450 µm) failed to support wrapping ECM. Finally, we demonstrate that vascular smooth muscle cells attached on ECFC-seeded microfibers, depositing collagen I and elastin. Collectively, we establish a novel in vitro model for the sequential control and study of microvasculature development and reveal the unprecedented role of the endothelium in organized ECM deposition regulated by the microfiber curvature.
Conflict of interest statement
Figures

References
-
- Jain RK (2003) Molecular regulation of vessel maturation. Nat Med 9: 685–693. - PubMed
-
- Carmeliet P, Jain RK (2000) Angiogenesis in cancer and other diseases. Nature 407: 249–257. - PubMed
-
- Hynes RO (2007) Cell–matrix adhesion in vascular development. JThromb Haemost 5: 32–40. - PubMed
-
- Ranjan A, Webster T (2009) Increased endothelial cell adhesion and elongation on micron-patterned nano-rough poly(dimethylsiloxane) films. Nanotechnology 20: 305102. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

