Highly sensitive in vivo imaging of Trypanosoma brucei expressing "red-shifted" luciferase
- PMID: 24278497
- PMCID: PMC3836995
- DOI: 10.1371/journal.pntd.0002571
Highly sensitive in vivo imaging of Trypanosoma brucei expressing "red-shifted" luciferase
Abstract
Background: Human African trypanosomiasis is caused by infection with parasites of the Trypanosoma brucei species complex, and threatens over 70 million people in sub-Saharan Africa. Development of new drugs is hampered by the limitations of current rodent models, particularly for stage II infections, which occur once parasites have accessed the CNS. Bioluminescence imaging of pathogens expressing firefly luciferase (emission maximum 562 nm) has been adopted in a number of in vivo models of disease to monitor dissemination, drug-treatment and the role of immune responses. However, lack of sensitivity in detecting deep tissue bioluminescence at wavelengths below 600 nm has restricted the wide-spread use of in vivo imaging to investigate infections with T. brucei and other trypanosomatids.
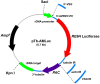
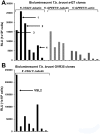
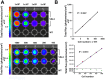
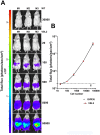
Methodology/principal findings: Here, we report a system that allows the detection of fewer than 100 bioluminescent T. brucei parasites in a murine model. As a reporter, we used a codon-optimised red-shifted Photinus pyralis luciferase (PpyRE9H) with a peak emission of 617 nm. Maximal expression was obtained following targeted integration of the gene, flanked by an upstream 5'-variant surface glycoprotein untranslated region (UTR) and a downstream 3'-tubulin UTR, into a T. brucei ribosomal DNA locus. Expression was stable in the absence of selective drug for at least 3 months and was not associated with detectable phenotypic changes. Parasite dissemination and drug efficacy could be monitored in real time, and brain infections were readily detectable. The level of sensitivity in vivo was significantly greater than achievable with a yellow firefly luciferase reporter.
Conclusions/significance: The optimised bioluminescent reporter line described here will significantly enhance the application of in vivo imaging to study stage II African trypanosomiasis in murine models. The greatly increased sensitivity provides a new framework for investigating host-parasite relationships, particularly in the context of CNS infections. It should be ideally suited to drug evaluation programmes.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
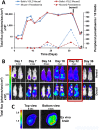

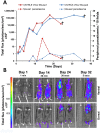
References
-
- WHO (2012) Human African Trypanosomiasis (sleeping sickness). Factsheet No 259.
-
- Wilkinson SR, Kelly JM (2009) Trypanocidal drugs: mechanisms, resistance and new targets. Expert Rev Mol Med 11: e31. - PubMed
-
- Barrett MP, Vincent IM, Burchmore RJ, Kazibwe AJ, Matovu E (2011) Drug resistance in human African trypanosomiasis. Future Microbiol 6: 1037–1047. - PubMed
-
- Checkley AM, Pepin J, Gibson WC, Taylor MN, Jager HR, et al. (2007) Human African trypanosomiasis: diagnosis, relapse and survival after severe melarsoprol-induced encephalopathy. Trans R Soc Trop Med Hyg 101: 523–526. - PubMed
-
- Pepin J, Milord F, Khonde AN, Niyonsenga T, Loko L, et al. (1995) Risk factors for encephalopathy and mortality during melarsoprol treatment of Trypanosoma brucei gambiense sleeping sickness. Trans R Soc Trop Med Hyg 89: 92–97. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

