RUNX2, GPX3 and PTX3 gene expression profiling in cumulus cells are reflective oocyte/embryo competence and potentially reliable predictors of embryo developmental competence in PCOS patients
- PMID: 24279306
- PMCID: PMC4222840
- DOI: 10.1186/1477-7827-11-109
RUNX2, GPX3 and PTX3 gene expression profiling in cumulus cells are reflective oocyte/embryo competence and potentially reliable predictors of embryo developmental competence in PCOS patients
Abstract
Background: Polycystic ovary syndrome (PCOS) is a common endocrine and metabolic disorder in women. The developmental competence of oocytes and embryos in PCOS patients is reduced to a certain extent (comparing to non-PCOS patients, the high quality embryo rate was decreased by 16% from the data of our centre) during the in vitro fertilization (IVF) process. Cross-talk between the oocyte and cumulus cells is critical for oocyte maturation and embryo competence. In this study, we have evaluated the transcription of specific genes in cumulus cells harvested from pre-ovulatory follicles of PCOS patients before IVF, according to individual oocyte nuclear maturity and developmental competence. Seven genes (RUNX2, PSAT1, ADAMTS9, CXCL1, CXCL2, CXCL3, and ITGB5) were targeted from our previous cDNA microarray data which isolated genes related to oocyte nuclear maturation in PCOS patients. Two additional genes which had been found to be associated with oocyte maturation or embryo quality in non-PCOS patients (GPX3 and PTX3) were also studied.
Methods: The mRNA expression levels of cumulus cells were detected by qRT- PCR.
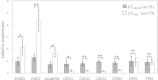
Results: Consistent with our previous cDNA microarray data, with the exception of GPX3 and PTX3, the selected 7 genes were related to oocyte nuclear maturation in PCOS patients. Noticeably, the expression level of RUNX2 was lower in cumulus cells derived from oocytes that could develop into blastocysts than the level of expression from oocytes that could not. The PTX3 expression level was significantly lower in cumulus cells from oocytes with two normal pronuclei than that from oocytes that formed >2 pronuclei (MPN) after fertilization. GPX3 mRNA levels were decreased in cumulus cells isolated from oocytes that developed into blastocysts with high potential development competence.
Conclusions: Several cumulus cell genes were associated with oocyte maturation, fertilization and embryo quality in PCOS patients. RUNX2 and GPX3 are candidate genetic markers in the monitoring of embryo quality for PCOS patients, whereas PTX3 mainly played a role in fertilization process. Together with morphological evaluation, cumulus cells genes may serve as biomarkers of oocyte and embryo selection during the IVF process for PCOS patients and may advance our understanding of PCOS.
Figures



Similar articles
-
Correlation of cumulus gene expression of GJA1, PRSS35, PTX3, and SERPINE2 with oocyte maturation, fertilization, and embryo development.Reprod Biol Endocrinol. 2015 Aug 16;13:93. doi: 10.1186/s12958-015-0091-3. Reprod Biol Endocrinol. 2015. PMID: 26276571 Free PMC article.
-
An improved IVM method for cumulus-oocyte complexes from small follicles in polycystic ovary syndrome patients enhances oocyte competence and embryo yield.Hum Reprod. 2017 Oct 1;32(10):2056-2068. doi: 10.1093/humrep/dex262. Hum Reprod. 2017. PMID: 28938744
-
Differences in the transcriptional profiles of human cumulus cells isolated from MI and MII oocytes of patients with polycystic ovary syndrome.Reproduction. 2013 May 21;145(6):597-608. doi: 10.1530/REP-13-0005. Print 2013 Jun. Reproduction. 2013. PMID: 23603633
-
PCOS and Role of Cumulus Gene Expression in Assessing Oocytes Quality.Front Endocrinol (Lausanne). 2022 May 26;13:843867. doi: 10.3389/fendo.2022.843867. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35721714 Free PMC article. Review.
-
CUMULUS CELL GENES AS POTENTIAL BIOMARKERS OF OOCYTE AND EMBRYO DEVELOPMENTAL COMPETENCE.Fiziol Zh (1994). 2016;62(1):107-13. doi: 10.15407/fz62.01.107. Fiziol Zh (1994). 2016. PMID: 29537212 Review.
Cited by
-
Comparative Effectiveness of Three Ovarian Hyperstimulation Protocol in In Vitro Fertilization (IVF) Cycles for Women with Polycystic Ovary Syndrome.Med Sci Monit. 2018 Dec 28;24:9424-9428. doi: 10.12659/MSM.913757. Med Sci Monit. 2018. PMID: 30591703 Free PMC article.
-
Oocyte-somatic cell interactions in the human ovary-novel role of bone morphogenetic proteins and growth differentiation factors.Hum Reprod Update. 2016 Dec;23(1):1-18. doi: 10.1093/humupd/dmw039. Epub 2016 Oct 26. Hum Reprod Update. 2016. PMID: 27797914 Free PMC article. Review.
-
Redox Biology of Human Cumulus Cells: Basic Concepts, Impact on Oocyte Quality, and Potential Clinical Use.Antioxid Redox Signal. 2020 Mar 10;32(8):522-535. doi: 10.1089/ars.2019.7984. Antioxid Redox Signal. 2020. PMID: 31861967 Free PMC article. Review.
-
The role of ADAMTS4 and ADAMTS9 in cardiovascular disease in premature ovarian insufficiency and idiopathic hypogonadotropic hypogonadism.J Endocrinol Invest. 2018 Dec;41(12):1477-1483. doi: 10.1007/s40618-018-0948-3. Epub 2018 Sep 5. J Endocrinol Invest. 2018. PMID: 30187439
-
Induction of proteinases in the human preovulatory follicle of the menstrual cycle by human chorionic gonadotropin.Fertil Steril. 2015 Mar;103(3):826-33. doi: 10.1016/j.fertnstert.2014.11.017. Epub 2014 Dec 13. Fertil Steril. 2015. PMID: 25516084 Free PMC article.
References
-
- Wood JR, Nelson VL, Ho C, Jansen E, Wang CY, Urbanek M, McAllister JM, Mosselman S, Strauss JF 3rd. The molecular phenotype of polycystic ovary syndrome (PCOS) theca cells and new candidate PCOS genes defined by microarray analysis. J Biol Chem. 2003;278:26380–26390. doi: 10.1074/jbc.M300688200. - DOI - PubMed
-
- Wood JR, Dumesic DA, Abbott DH, Strauss JF 3rd. Molecular abnormalities in oocytes from women with polycystic ovary syndrome revealed by microarray analysis. J Clin Endocrinol Metab. 2007;92:705–713. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

