Inhibition of phosphoinositol 3 kinase contributes to nanoparticle-mediated exaggeration of endotoxin-induced leukocyte procoagulant activity
- PMID: 24279459
- PMCID: PMC4035470
- DOI: 10.2217/nnm.13.137
Inhibition of phosphoinositol 3 kinase contributes to nanoparticle-mediated exaggeration of endotoxin-induced leukocyte procoagulant activity
Abstract
Aim: Disseminated intravascular coagulation is an increasing concern for certain types of engineered nanomaterials. Recent studies have shed some light on the nanoparticle physicochemical properties contributing to this toxicity; however, the mechanisms are poorly understood. Leukocyte procoagulant activity (PCA) is a key factor contributing to the initiation of this toxicity. We have previously reported on the exaggeration of endotoxin-induced PCA by cationic dendrimers. Herein, we report an effort to discern the mechanism.
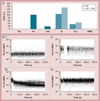
Materials & methods: Poly(amidoamine) dendrimers with various sizes and surface functionalities were studied in vitro by the recalcification test, flow cytometry and other relevant assays.
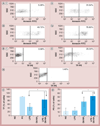
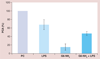
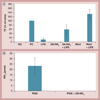
Results & conclusion: Cationic dendrimers exaggerated endotoxin-induced PCA, but their anionic or neutral counterparts did not; the cationic charge prompts this phenomenon, but different cationic surface chemistries do not influence it. Cationic dendrimers and endotoxin differentially affect the PCA complex. The inhibition of phosphoinositol 3 kinase by dendrimers contributes to the exaggeration of the endotoxin-induced PCA.
Keywords: coagulopathy; dendrimer; disseminated intravascular coagulation; leukocyte; nanoparticle; procoagulant activity; thrombosis.
Conflict of interest statement
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Figures



Similar articles
-
Dendrimer-induced leukocyte procoagulant activity depends on particle size and surface charge.Nanomedicine (Lond). 2012 Feb;7(2):245-56. doi: 10.2217/nnm.11.105. Epub 2011 Sep 30. Nanomedicine (Lond). 2012. PMID: 21957862
-
Rat blood leucocytes, unlike rabbit leucocytes, do not generate procoagulant activity on exposure to endotoxin.Br J Exp Pathol. 1981 Dec;62(6):638-42. Br J Exp Pathol. 1981. PMID: 7326218 Free PMC article.
-
Endotoxin-induced procoagulant activity in equine peripheral blood monocytes.Circ Shock. 1988 Nov;26(3):297-309. Circ Shock. 1988. PMID: 3208423
-
Toxicology of Engineered Nanoparticles: Focus on Poly(amidoamine) Dendrimers.Int J Environ Res Public Health. 2018 Feb 14;15(2):338. doi: 10.3390/ijerph15020338. Int J Environ Res Public Health. 2018. PMID: 29443901 Free PMC article. Review.
-
Interaction of nanoparticles with endotoxin Importance in nanosafety testing and exploitation for endotoxin binding.Nanotoxicology. 2021 May;15(4):558-576. doi: 10.1080/17435390.2021.1898690. Epub 2021 Mar 30. Nanotoxicology. 2021. PMID: 33784953 Review.
Cited by
-
Multicolor flow cytometry-based immunophenotyping for preclinical characterization of nanotechnology-based formulations: an insight into structure activity relationship and nanoparticle biocompatibility profiles.Front Allergy. 2023 Jul 4;4:1126012. doi: 10.3389/falgy.2023.1126012. eCollection 2023. Front Allergy. 2023. PMID: 37470031 Free PMC article.
-
Safety Challenges and Application Strategies for the Use of Dendrimers in Medicine.Pharmaceutics. 2022 Jun 17;14(6):1292. doi: 10.3390/pharmaceutics14061292. Pharmaceutics. 2022. PMID: 35745863 Free PMC article. Review.
-
Pre-clinical immunotoxicity studies of nanotechnology-formulated drugs: Challenges, considerations and strategy.J Control Release. 2015 Dec 28;220(Pt B):571-83. doi: 10.1016/j.jconrel.2015.08.056. Epub 2015 Sep 5. J Control Release. 2015. PMID: 26348388 Free PMC article. Review.
-
Subchronic and chronic toxicity evaluation of inorganic nanoparticles for delivery applications.Adv Drug Deliv Rev. 2019 Apr;144:112-132. doi: 10.1016/j.addr.2019.07.006. Epub 2019 Jul 8. Adv Drug Deliv Rev. 2019. PMID: 31295521 Free PMC article. Review.
-
Current understanding of interactions between nanoparticles and the immune system.Toxicol Appl Pharmacol. 2016 May 15;299:78-89. doi: 10.1016/j.taap.2015.12.022. Epub 2015 Dec 29. Toxicol Appl Pharmacol. 2016. PMID: 26739622 Free PMC article. Review.
References
-
- Levi M. Disseminated intravascular coagulation. Crit. Care Med. 2007;35(9):2191–2195. - PubMed
-
- Levi M, Schultz M, van der Poll T. Disseminated intravascular coagulation in infectious disease. Semin. Thromb. Hemost. 2010;36(4):367–377. - PubMed
-
- Franchini M, Di Minno MN, Coppola A. Disseminated intravascular coagulation in hematologic malignancies. Semin. Thromb. Hemost. 2010;36(4):388–403. - PubMed
-
- Lippi G, Cervellin G. Disseminated intravascular coagulation in trauma injuries. Semin. Thromb. Hemost. 2010;36(4):378–387. - PubMed
-
- Montagnana M, Franchi M, Danese E, Gotsch F, Guidi GC. Disseminated intravascular coagulation in obstetric and gynecologic disorders. Semin. Thromb. Hemost. 2010;36(4):404–418. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
