The effects of Eculizumab on the pathology of malignant atrophic papulosis
- PMID: 24279613
- PMCID: PMC3879088
- DOI: 10.1186/1750-1172-8-185
The effects of Eculizumab on the pathology of malignant atrophic papulosis
Abstract
Background: Degos disease is a frequently fatal and incurable occlusive vasculopathy most commonly affecting the skin, gastrointestinal tract and brain. Vascular C5b-9 deposition and a type I interferon (IFN) rich microenvironment are held to be pathogenetically important in the evolution of the vascular changes. We recently discovered the use of eculizumab as a salvage drug in the treatment of near fatal Malignant atrophic papulosis (MAP). The effects of eculizumab on the pathology of MAP are explored.
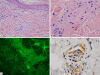
Methods: Archival skin and gastrointestinal biopsy material was procured over a 2.5-year period before and after eculizumab therapy in our index case. Routine light microscopy and immunohistochemical assessment for C3d, C4d, C5b-9, MxA and caspase 3 were examined. Direct immunofluorescent studies were also conducted on select biopsy material.
Results: The patient had received eculizumab as a emergent life saving measure and following rapid improvement he continued with biweekly infusions for 4 years. Although improved he continues to have signs and symptoms of persistent abdominal disease. Pre-Eculizumab biopsies showed an active thrombotic microangiopathy associated with a high type I interferon signature and extensive vascular deposits of C5b-9 in skin and gastrointestinal biopsies. Endothelial cell apoptosis as revealed by Caspase 3 expression was noted. Inflammation comprising lymphocytes and macrophages along with mesenchymal mucin was observed as well. Post-eculizumab biopsies did not show active luminal thrombosis but only chronic sequelae of prior episodes of vascular injury. There was no discernible caspase 3 expression. After 12 months of therapy, C5b-9 was no longer detectable in tissue. The high type I IFN signature and inflammation along with mucin deposition was not altered by the drug. In addition, there was little effect of the drug on the occlusive fibrointimal arteriopathy which appears to be one characterized by extensive myofibroblastic expansion of the intima potentially as revealed by staining for smooth muscle actin without immunoreactivity for desmin and myogenin.
Conclusions: Complement activation and enhanced endothelial cell apoptosis play an important role in the thrombotic complications of MAP. However, the larger vessel proliferative intimal changes appear to be independent of complement activation and may be on the basis of other upstream mechanisms. Monitoring C5b-9 deposition in tissue is likely not of great value in assessing treatment response to eculizumab given the persistence of C5b-9 in tissue for several months despite clinically effective C5 blocking therapy. A more integrated approach addressing upstream and downstream pathways in addition to those attributable to complement activation are critical for the successful treatment of MAP. Eculizumab may be used as salvage therapy in critically ill patients with thrombotic microangiopathy.
Figures






References
-
- Doutre MS, Beylot C, Bioulac P, Busquet M, Conte M. Skin lesion resembling malignant atrophic papulosis in lupus erythematosus. Dermatologica. 1987;8:45–46. - PubMed
-
- Notash AY, Mazoochy H, Mirshams M, Nikoo A. Lethal systemic Degos disease with prominent cardio-pulmonary involvement. Saudi Med J. 2008;8:133–137. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

