A randomized, controlled trial of everolimus-based dual immunosuppression versus standard of care in de novo kidney transplant recipients
- PMID: 24279685
- PMCID: PMC4282427
- DOI: 10.1111/tri.12252
A randomized, controlled trial of everolimus-based dual immunosuppression versus standard of care in de novo kidney transplant recipients
Abstract
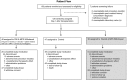
Kidney transplant recipients receiving calcineurin inhibitor-based immunosuppression incur increased long-term risks of cancer and kidney fibrosis. Switch to mammalian target of rapamycin (mTOR) inhibitors may reduce these risks. Steroid or Cyclosporin Removal After Transplant using Everolimus (SOCRATES), a 36-month, prospective, multinational, open-label, randomized controlled trial for de novo kidney transplant recipients, assessed whether everolimus switch could enable elimination of mycophenolate plus either steroids or CNI without compromising efficacy. Patients received cyclosporin, mycophenolate and steroids for the first 14 days then everolimus with mycophenolate and CNIwithdrawal (CNI-WD); everolimus with mycophenolate and steroid withdrawal (steroid-WD); or cyclosporin, mycophenolate and steroids (control). 126 patients were randomized. The steroid WD arm was terminated prematurely because of excess discontinuations. Mean eGFR at month 12 for CNI-WD versus control was 65.1 ml/min/1.73 m2 vs. 67.1 ml/min/1.73 m2 by ITT, which met predefined noninferiority criteria (P=0.026). The CNI-WD group experienced a higher rate of BPAR(31% vs. control 13%, P=0.048) and showed a trend towards higher composite treatment failure (BPAR, graft loss, death, loss to follow-up). The 12 month results from SOCRATES show noninferiority in eGFR, but a significant excess of acute rejection when everolimus was commenced at week 2 to enable a progressive withdrawal of mycophenolate and cyclosporin in kidney transplant recipients.
Keywords: cyclosporin; everolimus; kidney transplantation; mammalian target of rapamycin; steroids.
© 2013 The Authors. Transplant International published by John Wiley & Sons Ltd on behalf of Steunstichting ESOT.
Figures



References
-
- Vincenti F, Friman S, Scheuermann E, et al. Results of an international, randomized trial comparing glucose metabolism disorders and outcome with cyclosporine versus tacrolimus. Am J Transplant. 2007;7:1506. - PubMed
-
- Clayton P, Campbell S, Hurst K, et al. ANZDATA 2011 Annual report. Chapter 8 Transplantation. Available via: http://www.anzdata.org.au/v1/report_2011.html.
-
- Meier-Kriesche HU, Schold JD, Srinivas TR, et al. Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. Am J Transplant. 2004;4:378. - PubMed
-
- Nankivell BJ, Borrows RJ, Fung CL, et al. The natural history of chronic allograft Nephropathy. N Engl J Med. 2003;349:2326. - PubMed
-
- Pilmore H, Dent H, Chang S, et al. Reduction in cardiovascular death after kidney transplantation. Transplantation. 2010;89:851. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

