A comprehensive drug safety evaluation of pregabalin in peripheral neuropathic pain
- PMID: 24279736
- PMCID: PMC4320770
- DOI: 10.1111/papr.12146
A comprehensive drug safety evaluation of pregabalin in peripheral neuropathic pain
Abstract
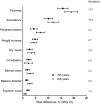
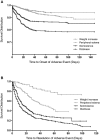
Pregabalin is a commonly used therapy currently recommended as first-line treatment for a number of neuropathic pain (NeP) conditions. Since licensure, a number of clinical trials of pregabalin in different NeP conditions have been completed from which additional data on safety and tolerability can be drawn. In this analysis, patient-level data from 31 randomized clinical trials of pregabalin in peripheral NeP sponsored by Pfizer were pooled and assessed for incidence of adverse events (AEs). Incidence by age, disease condition, and race, together with risk differences and time to onset and resolution of AEs, was assessed. In total, 7,510 patients were included: 4,884 on pregabalin (representing 805 patient-years treatment) and 2,626 on placebo. Pregabalin vs. placebo risk analysis identified 9 AEs with a risk difference, for which the lower limit of the 95% confidence interval (CI) was > 1%: dizziness (risk difference [95% CI]: (17.0 [15.4 to 18.6]), somnolence (10.8 [9.5 to 12.1]), peripheral edema (5.4 [4.3 to 6.4]), weight increase (4.7 [3.9 to 5.5]), dry mouth (2.9 [2.1 to 3.8]), constipation (2.3 [1.5 to 3.2]), blurred vision (2.2 [1.6 to 2.9]), balance disorder (2.0 [1.5 to 2.5]), and euphoric mood (1.6 [1.2 to 2.0]). The most common AEs, dizziness and somnolence, typically emerged within the first 1 to 2 weeks of treatment and resolved 1 to 2 weeks later, without resulting in cessation of treatment. The data from this review provide information, indicating which AEs may be expected in patients treated with pregabalin, and suggest that careful dose titration to the highest tolerable dose is the most appropriate approach in clinical practice.
Keywords: adverse events; diabetic; neuralgia; pain; peripheral neuropathic pain; postherpetic neuralgia; pregabalin; safety.
© 2013 Pfizer Inc. Pain Practice published by Wiley Periodicals, Inc. on behalf of World Institute of Pain.
Figures
References
-
- Bouhassira D, Lantéri-Minet M, Attal N, Laurent B, Touboul C. Prevalence of chronic pain with neuropathic characteristics in the general population. Pain. 2008;136:380–387. - PubMed
-
- Toth C. Drug safety evaluation of pregabalin. Expert Opin Drug Saf. 2012;11:487–502. - PubMed
-
- Freynhagen R, Bennett MI. Diagnosis and management of neuropathic pain. BMJ. 2009;339:b3002. - PubMed
-
- Bril V, England J, Franklin GM, et al. Evidence-based guideline: Treatment of painful diabetic neuropathy. Report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2011;76:1758–1765. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

