The ζ isoform of diacylglycerol kinase plays a predominant role in regulatory T cell development and TCR-mediated ras signaling
- PMID: 24280043
- PMCID: PMC4096120
- DOI: 10.1126/scisignal.2004373
The ζ isoform of diacylglycerol kinase plays a predominant role in regulatory T cell development and TCR-mediated ras signaling
Abstract
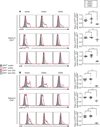
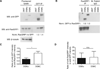
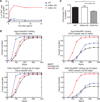
Diacylglycerol (DAG) is a critical second messenger that mediates T cell receptor (TCR)-stimulated signaling. The abundance of DAG is reduced by the diacylglycerol kinases (DGKs), which catalyze the conversion of DAG to phosphatidic acid (PA) and thus inhibit DAG-mediated signaling. In T cells, the predominant DGK isoforms are DGKα and DGKζ, and deletion of the genes encoding either isoform enhances DAG-mediated signaling. We found that DGKζ, but not DGKα, suppressed the development of natural regulatory T (T(reg)) cells and predominantly mediated Ras and Akt signaling downstream of the TCR. The differential functions of DGKα and DGKζ were not attributable to differences in protein abundance in T cells or in their localization to the contact sites between T cells and antigen-presenting cells. RasGRP1, a key DAG-mediated activator of Ras signaling, associated to a greater extent with DGKζ than with DGKα; however, in silico modeling of TCR-stimulated Ras activation suggested that a difference in RasGRP1 binding affinity was not sufficient to cause differences in the functions of each DGK isoform. Rather, the model suggested that a greater catalytic rate for DGKζ than for DGKα might lead to DGKζ exhibiting increased suppression of Ras-mediated signals compared to DGKα. Consistent with this notion, experimental studies demonstrated that DGKζ was more effective than DGKα at catalyzing the metabolism of DAG to PA after TCR stimulation. The enhanced effective enzymatic production of PA by DGKζ is therefore one possible mechanism underlying the dominant functions of DGKζ in modulating T(reg) cell development.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

