Activated lymphocytes as a metabolic model for carcinogenesis
- PMID: 24280044
- PMCID: PMC3834493
- DOI: 10.1186/2049-3002-1-5
Activated lymphocytes as a metabolic model for carcinogenesis
Abstract
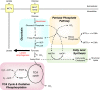
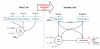
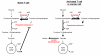
Metabolic reprogramming is a key event in tumorigenesis to support cell growth, and cancer cells frequently become both highly glycolytic and glutamine dependent. Similarly, T lymphocytes (T cells) modify their metabolism after activation by foreign antigens to shift from an energetically efficient oxidative metabolism to a highly glycolytic and glutamine-dependent metabolic program. This metabolic transition enables T cell growth, proliferation, and differentiation. In both activated T cells and cancer cells metabolic reprogramming is achieved by similar mechanisms and offers similar survival and cell growth advantages. Activated T cells thus present a useful model with which to study the development of tumor metabolism. Here, we review the metabolic similarities and distinctions between activated T cells and cancer cells, and discuss both the common signaling pathways and master metabolic regulators that lead to metabolic rewiring. Ultimately, understanding how and why T cells adopt a cancer cell-like metabolic profile may identify new therapeutic strategies to selectively target tumor metabolism or inflammatory immune responses.
Figures
References
-
- Khorana HG. Chemical Biology; Selected Papers of H. Gobind Khorana. Singapore: World Scientific Publishing Co; 2000.
LinkOut - more resources
Full Text Sources
Other Literature Sources

