Metabolic changes in cancer cells upon suppression of MYC
- PMID: 24280108
- PMCID: PMC4178210
- DOI: 10.1186/2049-3002-1-7
Metabolic changes in cancer cells upon suppression of MYC
Abstract
Background: Cancer cells engage in aerobic glycolysis and glutaminolysis to fulfill their biosynthetic and energetic demands in part by activating MYC. Previous reports have characterized metabolic changes in proliferating cells upon MYC loss or gain of function. However, metabolic differences between MYC-dependent cancer cells and their isogenic differentiated counterparts have not been characterized upon MYC suppression in vitro.
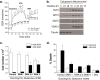
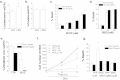
Results: Here we report metabolic changes between MYC-dependent mouse osteogenic sarcomas and differentiated osteoid cells induced upon MYC suppression. While osteogenic sarcoma cells increased oxygen consumption and spare respiratory capacity upon MYC suppression, they displayed minimal changes in glucose and glutamine consumption as well as their respective contribution to the citrate pool. However, glutamine significantly induced oxygen consumption in the presence of MYC which was dependent on aminotransferases. Furthermore, inhibition of aminotransferases selectively diminished cell proliferation and survival of osteogenic sarcoma MYC-expressing cells. There were minimal changes in ROS levels and cell death sensitivity to reactive oxygen species (ROS)-inducing agents between osteoid cells and osteogenic sarcoma cells. Nevertheless, the mitochondrial-targeted antioxidant Mito-Vitamin E still diminished proliferation of MYC-dependent osteogenic sarcoma cells.
Conclusion: These data highlight that aminotransferases and mitochondrial ROS might be attractive targets for cancer therapy in MYC-driven tumors.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

