Paradoxical abatement of striatal dopaminergic transmission by cocaine and methylphenidate
- PMID: 24280216
- PMCID: PMC3879549
- DOI: 10.1074/jbc.M113.495499
Paradoxical abatement of striatal dopaminergic transmission by cocaine and methylphenidate
Abstract
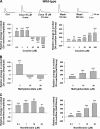
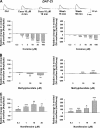
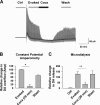
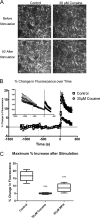
We combined in vitro amperometric, optical analysis of fluorescent false neurotransmitters and microdialysis techniques to unveil that cocaine and methylphenidate induced a marked depression of the synaptic release of dopamine (DA) in mouse striatum. In contrast to the classical dopamine transporter (DAT)-dependent enhancement of the dopaminergic signal observed at concentrations of cocaine lower than 3 μM, the inhibitory effect of cocaine was found at concentrations higher than 3 μM. The paradoxical inhibitory effect of cocaine and methylphenidate was associated with a decrease in synapsin phosphorylation. Interestingly, a cocaine-induced depression of DA release was only present in cocaine-insensitive animals (DAT-CI). Similar effects of cocaine were produced by methylphenidate in both wild-type and DAT-CI mice. On the other hand, nomifensine only enhanced the dopaminergic signal either in wild-type or in DAT-CI mice. Overall, these results indicate that cocaine and methylphenidate can increase or decrease DA neurotransmission by blocking reuptake and reducing the exocytotic release, respectively. The biphasic reshaping of DA neurotransmission could contribute to different behavioral effects of psychostimulants, including the calming ones, in attention deficit hyperactivity disorder.
Keywords: Amperometry; Cocaine; Dopamine; Dopamine Transporters; Drug Action; Electrophysiology; Microdialysis; Striatum; Transgenic Mice.
Figures


Similar articles
-
Alpha-synuclein/synapsin III pathological interplay boosts the motor response to methylphenidate.Neurobiol Dis. 2020 May;138:104789. doi: 10.1016/j.nbd.2020.104789. Epub 2020 Feb 4. Neurobiol Dis. 2020. PMID: 32032728
-
Serotonin transporter inhibition and 5-HT2C receptor activation drive loss of cocaine-induced locomotor activation in DAT Val559 mice.Neuropsychopharmacology. 2019 Apr;44(5):994-1006. doi: 10.1038/s41386-018-0301-8. Epub 2018 Dec 21. Neuropsychopharmacology. 2019. PMID: 30578419 Free PMC article.
-
Methylphenidate and cocaine self-administration produce distinct dopamine terminal alterations.Addict Biol. 2014 Mar;19(2):145-55. doi: 10.1111/j.1369-1600.2012.00456.x. Epub 2012 Mar 28. Addict Biol. 2014. PMID: 22458761 Free PMC article.
-
Neurobiological model of stimulated dopamine neurotransmission to interpret fast-scan cyclic voltammetry data.Brain Res. 2015 Mar 2;1599:67-84. doi: 10.1016/j.brainres.2014.12.020. Epub 2014 Dec 16. Brain Res. 2015. PMID: 25527399 Review.
-
Methylphenidate and cocaine: the same effects on gene regulation?Trends Pharmacol Sci. 2007 Nov;28(11):588-96. doi: 10.1016/j.tips.2007.10.004. Epub 2007 Oct 25. Trends Pharmacol Sci. 2007. PMID: 17963850 Review.
Cited by
-
Blunting neuroinflammation with resolvin D1 prevents early pathology in a rat model of Parkinson's disease.Nat Commun. 2019 Sep 2;10(1):3945. doi: 10.1038/s41467-019-11928-w. Nat Commun. 2019. PMID: 31477726 Free PMC article.
-
Accelerated development of cocaine-associated dopamine transients and cocaine use vulnerability following traumatic stress.Neuropsychopharmacology. 2020 Feb;45(3):472-481. doi: 10.1038/s41386-019-0526-1. Epub 2019 Sep 20. Neuropsychopharmacology. 2020. PMID: 31539899 Free PMC article.
-
Regulator of G protein signaling-12 modulates the dopamine transporter in ventral striatum and locomotor responses to psychostimulants.J Psychopharmacol. 2018 Feb;32(2):191-203. doi: 10.1177/0269881117742100. Epub 2018 Jan 24. J Psychopharmacol. 2018. PMID: 29364035 Free PMC article.
-
Ageing of skeletal muscle extracellular matrix and mitochondria: finding a potential link.Ann Med. 2023;55(2):2240707. doi: 10.1080/07853890.2023.2240707. Ann Med. 2023. PMID: 37643318 Free PMC article. Review.
-
Susceptibility to traumatic stress sensitizes the dopaminergic response to cocaine and increases motivation for cocaine.Neuropharmacology. 2017 Oct;125:295-307. doi: 10.1016/j.neuropharm.2017.07.032. Epub 2017 Aug 1. Neuropharmacology. 2017. PMID: 28778834 Free PMC article.
References
-
- Koob G. F., Bloom F. E. (1988) Cellular and molecular mechanisms of drug dependence. Science 242, 715–723 - PubMed
-
- Kuhar M. J., Ritz M. C., Boja J. W. (1991) The dopamine hypothesis of the reinforcing properties of cocaine. Trends Neurosci. 14, 299–302 - PubMed
-
- Chen R., Han D. D., Gu H. H. (2005) A triple mutation in the second transmembrane domain of mouse dopamine transporter markedly decreases sensitivity to cocaine and methylphenidate. J. Neurochem. 94, 352–359 - PubMed
-
- Penberthy J. K., Ait-Daoud N., Vaughan M., Fanning T. (2010) Review of treatment for cocaine dependence. Curr. Drug Abuse Rev. 3, 49–62 - PubMed
-
- Amalric M., Koob G. F. (1993) Functionally selective neurochemical afferents and efferents of the mesocorticolimbic and nigrostriatal dopamine system. Prog. Brain Res. 99, 209–226 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

