Assessing the need for and acceptability of a free-of-charge postpartum HPV vaccination program
- PMID: 24280248
- PMCID: PMC3995166
- DOI: 10.1016/j.ajog.2013.11.036
Assessing the need for and acceptability of a free-of-charge postpartum HPV vaccination program
Abstract
Objective: Human papillomavirus (HPV) vaccine uptake rate among young adult US women was only 23% in 2010. One way to improve this low rate is to administer the vaccine postpartum. We examined whether this population requires vaccination and whether they would be agreeable to receiving it free of charge after delivery.
Study design: Women 26 years of age or younger seeking prenatal care in publicly funded clinics in southeast Texas were interviewed in 2012 regarding their HPV vaccination status, barriers to vaccination, and whether they would be willing to receive this vaccine postpartum if offered free of charge. Medical charts were reviewed to extract additional information.
Results: Overall, 13.0% (65 of 500) stated they had initiated and 7.6% (38 of 500) completed the 3-dose vaccine series. Ethnic differences were noted with 21.0% of non-Hispanic whites, 14.6% of blacks, and 9.3% of Hispanics (P = .002) initiating the vaccine and 13.5%, 7.8%, and 5.2% (P = .006) competing all 3 doses, respectively. Lowest initiation (4.2%) and completion (1.4%) rates were observed among recently immigrated Hispanic women. Those who had not graduated from high school and older women were less likely to have been vaccinated. Almost 83% of those who had not received any HPV doses or completed the series were willing to receive the injection free of charge in the hospital after their delivery.
Conclusion: HPV vaccine uptake rates are very low among women receiving prenatal care in southeast Texas. Offering this vaccine free of charge to postpartum women could be an effective strategy in this population because 5 of 6 women favored receiving it in this setting.
Keywords: HPV; correlates; human papillomavirus; postpartum; race/ethnicity; vaccine.
Copyright © 2014 Mosby, Inc. All rights reserved.
Conflict of interest statement
The authors report no conflict of interest.
Figures
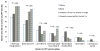
References
-
- Bosch FX, Burchell AN, Schiffman M, et al. Epidemiology and natural history of human papillomavirus infections and type-specific implications in cervical neoplasia. Vaccine. 2008;26:1–16. - PubMed
-
- Garland SM, Steben M, Sings HL, et al. Natural history of genital warts: analysis of placebo arm of 2 randomized phase III trials of quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine. J Infect Dis. 2009;199:805–14. - PubMed
-
- Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER. Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2007;56:1–24. - PubMed
-
- Centers for Disease Control and Prevention. FDA licensure of bivalent human papillomavirus vaccine (HPV2, Cervarix) for use in females and updated HPV vaccination recommendation from the Advisory Committee on Immunization Practices (ACIP) MMWR Morb Mortal Wkly Rep. 2010;59:626–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

