Pollen tube growth regulation by free anions depends on the interaction between the anion channel SLAH3 and calcium-dependent protein kinases CPK2 and CPK20
- PMID: 24280384
- PMCID: PMC3875734
- DOI: 10.1105/tpc.113.118463
Pollen tube growth regulation by free anions depends on the interaction between the anion channel SLAH3 and calcium-dependent protein kinases CPK2 and CPK20
Abstract
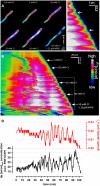
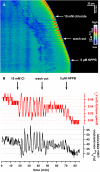
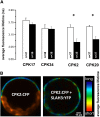
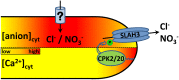
Apical growth in pollen tubes (PTs) is associated with the presence of tip-focused ion gradients and fluxes, implying polar localization or regulation of the underlying transporters. The molecular identity and regulation of anion transporters in PTs is unknown. Here we report a negative gradient of cytosolic anion concentration focused on the tip, in negative correlation with the cytosolic Ca(2+) concentration. We hypothesized that a possible link between these two ions is based on the presence of Ca(2+)-dependent protein kinases (CPKs). We characterized anion channels and CPK transcripts in PTs and analyzed their localization. Yellow fluorescent protein (YFP) tagging of a homolog of SLOW ANION CHANNEL-ASSOCIATED1 (SLAH3:YFP) was widespread along PTs, but, in accordance with the anion efflux, CPK2/CPK20/CPK17/CPK34:YFP fluorescence was strictly localized at the tip plasma membrane. Expression of SLAH3 with either CPK2 or CPK20 (but not CPK17/CPK34) in Xenopus laevis oocytes elicited S-type anion channel currents. Interaction of SLAH3 with CPK2/CPK20 (but not CPK17/CPK34) was confirmed by Förster-resonance energy transfer fluorescence lifetime microscopy in Arabidopsis thaliana mesophyll protoplasts and bimolecular fluorescence complementation in living PTs. Compared with wild-type PTs, slah3-1 and slah3-2 as well as cpk2-1 cpk20-2 PTs had reduced anion currents. Double mutant cpk2-1 cpk20-2 and slah3-1 PTs had reduced extracellular anion fluxes at the tip. Our studies provide evidence for a Ca(2+)-dependent CPK2/CPK20 regulation of the anion channel SLAH3 to regulate PT growth.
Figures




Similar articles
-
Tip-localized Ca2+ -permeable channels control pollen tube growth via kinase-dependent R- and S-type anion channel regulation.New Phytol. 2018 May;218(3):1089-1105. doi: 10.1111/nph.15067. Epub 2018 Mar 9. New Phytol. 2018. PMID: 29522235
-
Calcium-dependent protein kinases regulate polarized tip growth in pollen tubes.Plant J. 2009 Aug;59(4):528-39. doi: 10.1111/j.1365-313X.2009.03894.x. Epub 2009 Apr 11. Plant J. 2009. PMID: 19392698
-
An interconnection between tip-focused Ca2+ and anion homeostasis controls pollen tube growth.Plant Signal Behav. 2018;13(11):e1529521. doi: 10.1080/15592324.2018.1529521. Epub 2018 Oct 11. Plant Signal Behav. 2018. PMID: 30307369 Free PMC article.
-
Molecular identity of the outwardly rectifying, swelling-activated anion channel: time to reevaluate pICln.J Gen Physiol. 1998 May;111(5):617-22. doi: 10.1085/jgp.111.5.617. J Gen Physiol. 1998. PMID: 9565399 Free PMC article. Review. No abstract available.
-
The Role of Calcium/Calcium-Dependent Protein Kinases Signal Pathway in Pollen Tube Growth.Front Plant Sci. 2021 Mar 9;12:633293. doi: 10.3389/fpls.2021.633293. eCollection 2021. Front Plant Sci. 2021. PMID: 33767718 Free PMC article. Review.
Cited by
-
PbrSLAH3 is a nitrate-selective anion channel which is modulated by calcium-dependent protein kinase 32 in pear.BMC Plant Biol. 2019 May 8;19(1):190. doi: 10.1186/s12870-019-1813-z. BMC Plant Biol. 2019. PMID: 31068146 Free PMC article.
-
A mechanism of growth inhibition by abscisic acid in germinating seeds of Arabidopsis thaliana based on inhibition of plasma membrane H+-ATPase and decreased cytosolic pH, K+, and anions.J Exp Bot. 2015 Feb;66(3):813-25. doi: 10.1093/jxb/eru442. Epub 2014 Nov 4. J Exp Bot. 2015. PMID: 25371509 Free PMC article.
-
Calcium-Regulated Phosphorylation Systems Controlling Uptake and Balance of Plant Nutrients.Front Plant Sci. 2020 Feb 11;11:44. doi: 10.3389/fpls.2020.00044. eCollection 2020. Front Plant Sci. 2020. PMID: 32117382 Free PMC article. Review.
-
Kinase SnRK1.1 regulates nitrate channel SLAH3 engaged in nitrate-dependent alleviation of ammonium toxicity.Plant Physiol. 2021 May 27;186(1):731-749. doi: 10.1093/plphys/kiab057. Plant Physiol. 2021. PMID: 33560419 Free PMC article.
-
Chloride as a Beneficial Macronutrient in Higher Plants: New Roles and Regulation.Int J Mol Sci. 2019 Sep 21;20(19):4686. doi: 10.3390/ijms20194686. Int J Mol Sci. 2019. PMID: 31546641 Free PMC article. Review.
References
-
- Allen G.J., Chu S.P., Harrington C.L., Schumacher K., Hoffmann T., Tang Y.Y., Grill E., Schroeder J.I. (2001). A defined range of guard cell calcium oscillation parameters encodes stomatal movements. Nature 411: 1053–1057 - PubMed
-
- Allen G.J., Chu S.P., Schumacher K., Shimazaki C.T., Vafeados D., Kemper A., Hawke S.D., Tallman G., Tsien R.Y., Harper J.F., Chory J., Schroeder J.I. (2000). Alteration of stimulus-specific guard cell calcium oscillations and stomatal closing in Arabidopsis det3 mutant. Science 289: 2338–2342 - PubMed
-
- Benkert R., Obermeyer G., Bentrup F.-W. (1997). The turgor pressure of growing lily pollen tubes. Protoplasma 198: 1–8
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

