Quinoline 3-sulfonamides inhibit lactate dehydrogenase A and reverse aerobic glycolysis in cancer cells
- PMID: 24280423
- PMCID: PMC4178217
- DOI: 10.1186/2049-3002-1-19
Quinoline 3-sulfonamides inhibit lactate dehydrogenase A and reverse aerobic glycolysis in cancer cells
Abstract
Background: Most normal cells in the presence of oxygen utilize glucose for mitochondrial oxidative phosphorylation. In contrast, many cancer cells rapidly convert glucose to lactate in the cytosol, a process termed aerobic glycolysis. This glycolytic phenotype is enabled by lactate dehydrogenase (LDH), which catalyzes the inter-conversion of pyruvate and lactate. The purpose of this study was to identify and characterize potent and selective inhibitors of LDHA.
Methods: High throughput screening and lead optimization were used to generate inhibitors of LDHA enzymatic activity. Effects of these inhibitors on metabolism were evaluated using cell-based lactate production, oxygen consumption, and 13C NMR spectroscopy assays. Changes in comprehensive metabolic profile, cell proliferation, and apoptosis were assessed upon compound treatment.
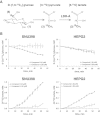
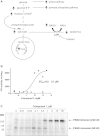
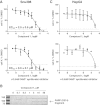
Results: 3-((3-carbamoyl-7-(3,5-dimethylisoxazol-4-yl)-6-methoxyquinolin-4-yl) amino) benzoic acid was identified as an NADH-competitive LDHA inhibitor. Lead optimization yielded molecules with LDHA inhibitory potencies as low as 2 nM and 10 to 80-fold selectivity over LDHB. Molecules in this family rapidly and profoundly inhibited lactate production rates in multiple cancer cell lines including hepatocellular and breast carcinomas. Consistent with selective inhibition of LDHA, the most sensitive breast cancer cell lines to lactate inhibition in hypoxic conditions were cells with low expression of LDHB. Our inhibitors increased rates of oxygen consumption in hepatocellular carcinoma cells at doses up to 3 microM, while higher concentrations directly inhibited mitochondrial function. Analysis of more than 500 metabolites upon LDHA inhibition in Snu398 cells revealed that intracellular concentrations of glycolysis and citric acid cycle intermediates were increased, consistent with enhanced Krebs cycle activity and blockage of cytosolic glycolysis. Treatment with these compounds also potentiated PKM2 activity and promoted apoptosis in Snu398 cells.
Conclusions: Rapid chemical inhibition of LDHA by these quinoline 3-sulfonamids led to profound metabolic alterations and impaired cell survival in carcinoma cells making it a compelling strategy for treating solid tumors that rely on aerobic glycolysis for survival.
Figures




Similar articles
-
Double genetic disruption of lactate dehydrogenases A and B is required to ablate the "Warburg effect" restricting tumor growth to oxidative metabolism.J Biol Chem. 2018 Oct 12;293(41):15947-15961. doi: 10.1074/jbc.RA118.004180. Epub 2018 Aug 29. J Biol Chem. 2018. PMID: 30158244 Free PMC article.
-
Stable shRNA Silencing of Lactate Dehydrogenase A (LDHA) in Human MDA-MB-231 Breast Cancer Cells Fails to Alter Lactic Acid Production, Glycolytic Activity, ATP or Survival.Anticancer Res. 2017 Mar;37(3):1205-1212. doi: 10.21873/anticanres.11435. Anticancer Res. 2017. PMID: 28314283 Free PMC article.
-
Inhibition of LDHA suppresses cell proliferation and increases mitochondrial apoptosis via the JNK signaling pathway in cervical cancer cells.Oncol Rep. 2022 Apr;47(4):77. doi: 10.3892/or.2022.8288. Epub 2022 Feb 22. Oncol Rep. 2022. PMID: 35191522 Free PMC article.
-
Role of LDH in tumor glycolysis: Regulation of LDHA by small molecules for cancer therapeutics.Semin Cancer Biol. 2022 Dec;87:184-195. doi: 10.1016/j.semcancer.2022.11.007. Epub 2022 Nov 9. Semin Cancer Biol. 2022. PMID: 36371026 Review.
-
Unappreciated Role of LDHA and LDHB to Control Apoptosis and Autophagy in Tumor Cells.Int J Mol Sci. 2019 Apr 27;20(9):2085. doi: 10.3390/ijms20092085. Int J Mol Sci. 2019. PMID: 31035592 Free PMC article. Review.
Cited by
-
The Regulation and Function of Lactate Dehydrogenase A: Therapeutic Potential in Brain Tumor.Brain Pathol. 2016 Jan;26(1):3-17. doi: 10.1111/bpa.12299. Epub 2015 Sep 17. Brain Pathol. 2016. PMID: 26269128 Free PMC article. Review.
-
Targeting Energy Metabolism in Cancer Treatment.Int J Mol Sci. 2022 May 16;23(10):5572. doi: 10.3390/ijms23105572. Int J Mol Sci. 2022. PMID: 35628385 Free PMC article. Review.
-
Functional inhibition of lactate dehydrogenase suppresses pancreatic adenocarcinoma progression.Clin Transl Med. 2021 Jun;11(6):e467. doi: 10.1002/ctm2.467. Clin Transl Med. 2021. PMID: 34185423 Free PMC article.
-
Targeting metabolism to enhance immunotherapy within tumor microenvironment.Acta Pharmacol Sin. 2024 Oct;45(10):2011-2022. doi: 10.1038/s41401-024-01304-w. Epub 2024 May 29. Acta Pharmacol Sin. 2024. PMID: 38811773 Review.
-
Discovery and Optimization of Potent, Cell-Active Pyrazole-Based Inhibitors of Lactate Dehydrogenase (LDH).J Med Chem. 2017 Nov 22;60(22):9184-9204. doi: 10.1021/acs.jmedchem.7b00941. Epub 2017 Nov 9. J Med Chem. 2017. PMID: 29120638 Free PMC article.
References
-
- Kolev Y, Uetake H, Takagi Y, Sugihara K. Lactate dehydrogenase-5 (LDH-5) expression in human gastric cancer: association with hypoxia-inducible factor (HIF-1alpha) pathway, angiogenic factors production and poor prognosis. Ann Surg Oncol. 2008;15:2336–2344. doi: 10.1245/s10434-008-9955-5. - DOI - PubMed
-
- Koukourakis MI, Giatromanolaki A, Sivridis E, Bougioukas G, Didilis V, Gatter KC, Harris AL. Lactate dehydrogenase-5 (LDH-5) overexpression in non-small-cell lung cancer tissues is linked to tumour hypoxia, angiogenic factor production and poor prognosis. Br J Cancer. 2003;89:877–885. doi: 10.1038/sj.bjc.6601205. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

