Insulin signaling, resistance, and the metabolic syndrome: insights from mouse models into disease mechanisms
- PMID: 24281010
- PMCID: PMC4087161
- DOI: 10.1530/JOE-13-0327
Insulin signaling, resistance, and the metabolic syndrome: insights from mouse models into disease mechanisms
Abstract
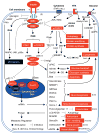
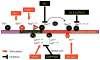
Insulin resistance is a major underlying mechanism responsible for the 'metabolic syndrome', which is also known as insulin resistance syndrome. The incidence of the metabolic syndrome is increasing at an alarming rate, becoming a major public and clinical problem worldwide. The metabolic syndrome is represented by a group of interrelated disorders, including obesity, hyperglycemia, hyperlipidemia, and hypertension. It is also a significant risk factor for cardiovascular disease and increased morbidity and mortality. Animal studies have demonstrated that insulin and its signaling cascade normally control cell growth, metabolism, and survival through the activation of MAPKs and activation of phosphatidylinositide-3-kinase (PI3K), in which the activation of PI3K associated with insulin receptor substrate 1 (IRS1) and IRS2 and subsequent Akt→Foxo1 phosphorylation cascade has a central role in the control of nutrient homeostasis and organ survival. The inactivation of Akt and activation of Foxo1, through the suppression IRS1 and IRS2 in different organs following hyperinsulinemia, metabolic inflammation, and overnutrition, may act as the underlying mechanisms for the metabolic syndrome in humans. Targeting the IRS→Akt→Foxo1 signaling cascade will probably provide a strategy for therapeutic intervention in the treatment of type 2 diabetes and its complications. This review discusses the basis of insulin signaling, insulin resistance in different mouse models, and how a deficiency of insulin signaling components in different organs contributes to the features of the metabolic syndrome. Emphasis is placed on the role of IRS1, IRS2, and associated signaling pathways that are coupled to Akt and the forkhead/winged helix transcription factor Foxo1.
References
-
- Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. - PubMed
-
- Accili D, Drago J, Lee EJ, Johnson MD, Cool MH, Salvatore P, Asico LD, Jose PA, Taylor SI, Westphal H. Early neonatal death in mice homozygous for a null allele of the insulin receptor gene. Nat Genet. 1996;12:106–109. - PubMed
-
- Alberti KG, Zimmet P, Shaw J. The metabolic syndrome--a new worldwide definition. Lancet. 2005;366:1059–1062. - PubMed
-
- Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. - PubMed
-
- Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol. 1997;7:261–269. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous