The role of oxidative stress in carcinogenesis induced by metals and xenobiotics
- PMID: 24281075
- PMCID: PMC3835083
- DOI: 10.3390/cancers2020376
The role of oxidative stress in carcinogenesis induced by metals and xenobiotics
Abstract
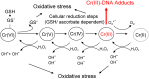
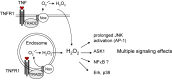
In addition to a wide range of adverse effects on human health, toxic metals such as cadmium, arsenic and nickel can also promote carcinogenesis. The toxicological properties of these metals are partly related to generation of reactive oxygen species (ROS) that can induce DNA damage and trigger redox-dependent transcription factors. The precise mechanisms that induce oxidative stress are not fully understood. Further, it is not yet known whether chronic exposures to low doses of arsenic, cadmium or other metals are sufficient to induce mutations in vivo, leading to DNA repair responses and/or tumorigenesis. Oxidative stress can also be induced by environmental xenobiotics, when certain metabolites are generated that lead to the continuous release of superoxide, as long as the capacity to reduce the resulting dions (quinones) into hydroquinones is maintained. However, the specific significance of superoxide-dependent pathways to carcinogenesis is often difficult to address, because formation of DNA adducts by mutagenic metabolites can occur in parallel. Here, we will review both mechanisms and toxicological consequences of oxidative stress triggered by metals and dietary or environmental pollutants in general. Besides causing DNA damage, ROS may further induce multiple intracellular signaling pathways, notably NF-kB, JNK/SAPK/p38, as well as Erk/MAPK. These signaling routes can lead to transcriptional induction of target genes that could promote proliferation or confer apoptosis resistance to exposed cells. The significance of these additional modes depends on tissue, cell-type and is often masked by alternate oncogenic mechanisms being activated in parallel.
Figures

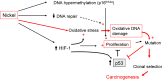
 ). An interplay of enhanced proliferation and up-regulation of p53 could constitute a strong selective pressure, favouring mutations, which may inactivate tumor suppressor genes (see text for details).
). An interplay of enhanced proliferation and up-regulation of p53 could constitute a strong selective pressure, favouring mutations, which may inactivate tumor suppressor genes (see text for details).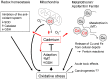
References
-
- Halliwell S., Gutteridge J.M.C. Free Radicals in Biology and Medicine. 4th ed. Oxford University Press; New York, NY, USA: 2007.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

